Iron
2007 Schools Wikipedia Selection. Related subjects: Chemical elements; Geology and geophysics
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
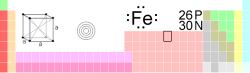
| Name, Symbol, Number | iron, Fe, 26 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 8, 4, d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous metallic with a grayish tinge  |
||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 55.845 (2) g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d6 4s2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 14, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.86 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 6.98 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1811 K (1538 ° C, 2800 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3134 K (2861 ° C, 5182 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.81 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 340 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 25.10 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic a=286.65 pm ; face-centered cubic between 1185–1667 K |
||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 3, 4, 6 ( amphoteric oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.83 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 762.5 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1561.9 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2957 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 140 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 156 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 125 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 96.1 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 80.4 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 11.8 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) (electrolytic) 5120 m·s−1 |
||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 211 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 82 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.29 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 608 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 490 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7439-89-6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
Iron ( IPA: /ˈʌɪə(r)n/) is a chemical element with the symbol Fe ( Latin: ferrum) and atomic number 26. Iron is a group 8 and period 4 metal. Iron and nickel are notable for being the final elements produced by stellar nucleosynthesis, and thus the heaviest elements which do not require a supernova or similarly cataclysmic event for formation. Iron and nickel are therefore the most abundant metals in metallic meteorites and in the dense-metal cores of planets such as Earth.
Notable characteristics
It is believed that iron is the tenth most abundant element in the universe. Iron makes up 5% of the Earth's crust and is second in abundance to aluminium among the metals and fourth in abundance among the elements. Iron is also the most abundant element by mass, making up 35% of the mass of the Earth as a whole. The concentration of iron in the various layers of the Earth ranges from very high at the inner core to only a few percent in the outer crust.
Iron is a metal extracted from iron ore, and is almost never found in the free elemental state. In order to obtain elemental iron, the impurities must be removed by chemical reduction. Iron is used in the production of steel, an alloy or solid solution of different metals, and some non-metals, particularly carbon. The many iron-carbon allotropes, which have very different properties, are discussed in the article on steel.
Nuclei of iron have some of the highest binding energies per nucleon, surpassed only by the nickel isotope 62Ni. The universally most abundant of the highly stable nucleides is, however, 56Fe. This is formed by nuclear fusion in the stars. Although a further tiny energy gain could be extracted by synthesizing 62Ni, conditions in stars are not right for this process to be favoured, and iron abundance on Earth greatly favors iron over nickel, and also presumably in supernova element production. When a very large star contracts at the end of its life, internal pressure and temperature rise, allowing the star to produce progressively heavier elements, despite these being less stable than the elements around mass number 60, known as the "iron group". This leads to a supernova. Some cosmological models with an open universe predict that there will be a phase where as a result of slow fusion and fission reactions, everything will become iron.
Iron (as Fe2+, ferrous ion) is a necessary trace element used by all known living organisms. Iron-containing enzymes, usually containing heme prosthetic groups, participate in catalysis of oxidation reactions in biology, and in transport of a number of soluble gases. See hemoglobin, cytochrome, and catalase.
Applications
Iron is the most used of all the metals, comprising 95% of all the metal tonnage produced worldwide. Its combination of low cost and high strength make it indispensable, especially in applications like automobiles, the hulls of large ships, and structural components for buildings. Steel is the best known alloy of iron, and some of the forms that iron can take include:
- Pig iron has 4% – 5% carbon and contains varying amounts of contaminants such as sulfur, silicon and phosphorus. Its only significance is that of an intermediate step on the way from iron ore to cast iron and steel.
- Cast iron contains 2% – 4.0% carbon , 1% – 6% silicon , and small amounts of manganese. Contaminants present in pig iron that negatively affect the material properties, such as sulfur and phosphorus, have been reduced to an acceptable level. It has a melting point in the range of 1420–1470 K, which is lower than either of its two main components, and makes it the first product to be melted when carbon and iron are heated together. Its mechanical properties vary greatly, dependent upon the form carbon takes in the alloy. 'White' cast irons contain their carbon in the form of cementite, or iron carbide. This hard, brittle compound dominates the mechanical properties of white cast irons, rendering them hard, but unresistant to shock. The broken surface of a white cast iron is full of fine facets of the broken carbide, a very pale, silvery, shiny material, hence the appellation. In grey iron, the carbon exists free as fine flakes of graphite , and also, renders the material brittle due to the stress-raising nature of the sharp edged flakes of graphite. A newer variant of grey iron, referred to as ductile iron is specially treated with trace amounts of magnesium to alter the shape of graphite to sheroids, or nodules, vastly increasing the toughness and strength of the material.
- Carbon steel contains between 0.4% and 1.5% carbon, with small amounts of manganese, sulfur, phosphorus, and silicon.
- Wrought iron contains less than 0.2% carbon. It is a tough, malleable product, not as fusible as pig iron. It has a very small amount of carbon, a few tenths of a percent. If honed to an edge, it loses it quickly. Wrought iron is characterised, especially in old samples, by the presence of fine 'stringers' or filaments of slag entrapped in the metal. Wrought iron does not rust particularly quickly when used outdoors. It has largely been replaced by mild steel for "wrought iron" gates and blacksmithing. Mild steel does not have the same corrosion resistance but is cheaper and more widely available.
- Alloy steels contain varying amounts of carbon as well as other metals, such as chromium, vanadium, molybdenum, nickel, tungsten, etc. They are used for structural purposes, as their alloy content raises their cost and necessitates justification of their use. Recent developments in ferrous metallurgy have produced a growing range of microalloyed steels, also termed 'HSLA' or high-strength, low alloy steels, containing tiny additions to produce high strengths and often spectacular toughness at minimal cost.
- Iron(III) oxides are used in the production of magnetic storage media in computers. They are often mixed with other compounds, and retain their magnetic properties in solution.
The main drawback to iron and steel is that pure iron, and most of its alloys, suffer badly from rust if not protected in some way. Painting, galvanization, plastic coating and bluing are some techniques used to protect iron from rust by excluding water and oxygen or by sacrificial protection.
History
The first signs of use of iron come from the Sumerians and the Egyptians, where around 4000 BCE [citation needed], a few items, such as the tips of spears, daggers and ornaments, were being fashioned from iron recovered from meteorites [citation needed]. Because meteorites fall from the sky, some linguists have conjectured that the English word iron (OE īsern), which has cognates in many northern and western European languages, derives from the Etruscan aisar which means "the gods". Even if this is not the case, the word is likely a loan into pre- Proto-Germanic from Celtic or Italic (Krahe IF 46:184f. compares Old Irish, Illyrian, Venetic and Messapic forms). The meteoric origin of Iron in its first use by humans is also alluded to in the Quran : "and We sent down Iron in which is incredible strength and many benefits for mankind" (57:25 page:541)[linguistic citations needed; no mention of Proto-Boreal, Nostratic terms or Afro-Asiatic terms].
Ancient Greeks considered the Halybes to be "the inventors of iron" [citation needed; "the Greeks" are not a homogeneous group of people publishing one idea]. The people of the Caucasian Isthmus, Khaldi people (or Khalib/Halyb and Halisones by Strabo) were one of the oldest west-Georgian tribes (4th to 2nd millennia BC). The word "Halybes" may refer to people in Anatolia or in the Caucasus, and it is also possible that by the time the Greeks knew of iron, it was associated with Chaldea, where it was produced in large quantities (but not where it was invented).
By 2500 BCE to 2000 BCE, increasing numbers of smelted iron objects (distinguishable from meteoric iron by the lack of nickel in the product) appear in Mesopotamia, Anatolia, and Egypt[citation needed]. However, their use appears to be ceremonial, and iron was an expensive metal, more expensive than gold[citation needed, especially as regards to pricing concerning gold, as the word is often confused with "bronz" in ancient texts]. In the Iliad, weaponry is mostly bronze, but iron ingots are used for trade [an actual iron macehead was located at Troy in 1902; citation for either of these facts needed]. Some resources (see the reference What Caused the Iron Age? below) suggest that iron was being created then as a by-product of copper refining, as sponge iron, and was not reducible by the metallurgy of the time. By 1600 BCE to 1200 BCE, iron was used increasingly in the Middle East, but did not supplant the dominant use of bronze[citation needed; same story told about off-products of gold producing unusable iron].
In the period from the 12th to 10th century BCE, there was a rapid transition in the Middle East from bronze to iron tools and weapons. The critical factor in this transition does not appear to be the sudden onset of a superior iron working technology, but instead the disruption of the supply of tin. This period of transition, which occurred at different times in different parts of the world, is the ushering in of an age of civilization called the Iron Age. Classical authors ascribe the first invention of ironsmithing to peoples of the Caucasus and eastern Anatolia, such as the Khaldi (Chaldei) and the Khalib (Chalybes)[very likely the same people mentioned above - but erroneously located in Georgia, instead! the Khaldi are either in Georgia or Anatolia - but if both, then some major disambiguation needs to occur here]. If local customs regarding the importation of other metal-working techniques prevailed in the case of iron, then it would have been customary for people from the ironworking region (in this case, in Anatolia - since there are many agreements on that source), to establish self-named ethnic enclaves or new towns near the places where they wished to market their goods. Fierce defense of trade secrets had already made this the typical plan, whether with pottery, copper working, jewelry making or bronze making. The inventors moved closer to the markets, but kept themselves distinct and protected their secrets carefully. It is possible that other people came to these new metalworking towns to learn trade secrets, but probably had to pay a price of some kind (Abram and Lot may have been two such, coming to the new place, Ur of the Chaldees, which was a new Ur, not the old Ur, designed for metal production).
Concurrent with the transition from bronze to iron was the discovery of carburization, which was the process of adding carbon to the irons of the time. Iron was recovered as sponge iron, a mix of iron and slag with some carbon and/or carbide, which was then repeatedly hammered and folded over to free the mass of slag and oxidise out carbon content, so creating the product wrought iron. Wrought iron was very low in carbon content and was not easily hardened by quenching. The people of the Middle East found that a much harder product could be created by the long term heating of a wrought iron object in a bed of charcoal, which was then quenched in water or oil. The resulting product, which had a surface of steel, was harder and less brittle than the bronze it began to replace.
In China the first irons used were also meteoric iron, with archaeological evidence for items made of wrought iron appearing in the northwest, near Xinjiang, in the 8th century BCE. These items were made of wrought iron, created by the same processes used in the Middle East and Europe, and were thought to be imported by non-Chinese people.
In the later years of the Zhou Dynasty (ca 550 BCE), a new iron manufacturing capability began because of a highly developed kiln technology. Producing blast furnaces capable of temperatures exceeding 1300 K, the Chinese developed the manufacture of cast, or pig iron.
Iron was used in India as early as 250 BCE. The famous iron pillar in the Qutb complex in Delhi is made of very pure iron (98%) and has not rusted or eroded till this day.
If iron ores are heated with carbon to 1420–1470 K, a molten liquid is formed, an alloy of about 96.5% iron and 3.5% carbon. This product is strong, can be cast into intricate shapes, but is too brittle to be worked, unless the product is decarburized to remove most of the carbon. The vast majority of Chinese iron manufacture, from the Zhou dynasty onward, was of cast iron. Iron, however, remained a pedestrian product, used by farmers for hundreds of years, and did not really affect the nobility of China until the Qin dynasty (ca 221 BCE).
Cast iron development lagged in Europe, as the smelters could only achieve temperatures of about 1000 C; or perhaps they did not want hotter temperatures, as they were seeking to produce blooms as a precursor of wrought iron, not cast iron. Through a good portion of the Middle Ages, in Western Europe, iron was thus still being made by the working of iron blooms into wrought iron. Some of the earliest casting of iron in Europe occurred in Sweden, in two sites, Lapphyttan and Vinarhyttan, between 1150 and 1350 CE. Cast iron was then made into wrought iron by the osmond process. Some scholars have speculated the practice followed the Mongols across Russia to these sites, but there is no clear proof of this hypothesis. In any event, by the late fourteenth century, a market for cast iron goods began to form, as a demand developed for cast iron cannonballs.
Early iron smelting used charcoal as both the heat source and the reducing agent. In 18th century England, wood supplies became inadequate to enable the industry to expand and coke, a fossil fuel, began to be used an alternative. This innovation is associated with Abraham Darby at Coalbrookdale in 1709, but it was only later in the century that economically viable means of converting pig iron to bar iron were devised. The most successful such process was Henry Cort's puddling process, patented in 1784. Those processes permitted the great expansion in the production of iron that constitutes the Industrial Revolution for that industry.
Occurrence
Iron is one of the most common elements on Earth, making up about 5% of the Earth's crust. Most of this iron is found in various iron oxides, such as the minerals hematite, magnetite, and taconite. The earth's core is believed to consist largely of a metallic iron-nickel alloy. About 5% of the meteorites similarly consist of iron-nickel alloy. Although rare, these are the major form of natural metallic iron on the earth's surface.
Production of iron from iron ore

Industrially, iron is produced starting from iron ores, principally hematite (nominally Fe2O3) and magnetite (Fe3O4) by a carbothermic reaction (reduction with carbon) in a blast furnace at temperatures of about 2000 °C. In a blast furnace, iron ore, carbon in the form of coke, and a flux such as limestone are fed into the top of the furnace, while a blast of heated air is forced into the furnace at the bottom.
In the furnace, the coke reacts with oxygen in the air blast to produce carbon monoxide:
The carbon monoxide reduces the iron ore (in the chemical equation below, hematite) to molten iron, becoming carbon dioxide in the process:
- 6 CO + 2 Fe2O3 → 4 Fe + 6 CO2
The flux is present to melt impurities in the ore, principally silicon dioxide sand and other silicates. Common fluxes include limestone (principally calcium carbonate) and dolomite ( magnesium carbonate). Other fluxes may be used depending on the impurities that need to be removed from the ore. In the heat of the furnace the limestone flux decomposes to calcium oxide (quicklime):
- CaCO3 → CaO + CO2
Then calcium oxide combines with silicon dioxide to form a slag.
- CaO + SiO2 → CaSiO3
The slag melts in the heat of the furnace, which silicon dioxide would not have. In the bottom of the furnace, the molten slag floats on top of the more dense molten iron, and spouts in the side of the furnace may be opened to drain off either the iron or the slag. The iron once cooled, is called pig iron, while the slag can be used as a material in road construction or to improve mineral-poor soils for agriculture. Pig iron is later reduced to steel using convertors.
Approximately 1100Mt (million tons) of iron ore was produced in the world in 2000, with a gross market value of approximately 25 billion US dollars. While ore production occurs in 48 countries, the five largest producers were China, Brazil, Australia, Russia and India, accounting for 70% of world iron ore production. The 1100Mt of iron ore was used to produce approximately 572Mt of pig iron.
Isotopes
Naturally occurring iron consists of four isotopes: 5.845% of radioactive 54Fe (half-life: >3.1×1022 years), 91.754% of stable 56Fe, 2.119% of stable 57Fe and 0.282% of stable 58Fe. 60Fe is an extinct radionuclide of long half-life (1.5 million years). Much of the past work on measuring the isotopic composition of Fe has centered on determining 60Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation.
The isotope 56Fe is of particular interest to nuclear scientists. A common misconception is that this isotope represents the most stable nucleus possible, and that it thus would be impossible to perform fission or fusion on 56Fe and still liberate energy. This is not true, as both 62Ni and 58Fe are more stable.
In phases of the meteorites Semarkona and Chervony Kut a correlation between the concentration of 60Ni, the daughter product of 60Fe, and the abundance of the stable iron isotopes could be found which is evidence for the existence of 60Fe at the time of formation of the solar system. Possibly the energy released by the decay of 60Fe contributed, together with the energy released by decay of the radionuclide 26Al, to the remelting and differentiation of asteroids after their formation 4.6 billion years ago. The abundance of 60Ni present in extraterrestrial material may also provide further insight into the origin of the solar system and its early history. Of the stable isotopes, only 57Fe has a nuclear spin (−1/2).
Iron in biology
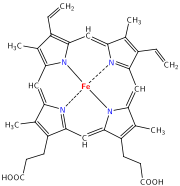
Iron is essential to all known organisms. It is mostly stably incorporated in the inside of metalloproteins, because in exposed or in free form it causes production of free radicals that are generally toxic to cells. To say that iron is free doesn't mean that it is free floating in the bodily fluids. Iron binds avidly to virtually all biomolecules so it will adhere nonspecifically to cell membranes, nucleic acids, proteins etc.
Many animals incorporate iron into the heme complex, an essential component of cytochromes, which are proteins involved in redox reactions (including but not limited to cellular respiration), and of oxygen carrying proteins hemoglobin and myoglobin. Inorganic iron involved in redox reactions is also found in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. A class of non-heme iron proteins is responsible for a wide range of functions within several life forms, such as enzymes methane monooxygenase (oxidizes methane to methanol), ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis), hemerythrins (oxygen transport and fixation in marine invertebrates) and purple acid phosphatase ( hydrolysis of phosphate esters). When the body is fighting a bacterial infection, the body sequesters iron inside of cells (mostly stored in the storage molecule ferritin) so that it cannot be used by bacteria.
Iron distribution is heavily regulated in mammals, both as a defense against bacterial infection and because of the potential biological toxicity of iron. The iron absorbed from the duodenum binds to transferrin, and is carried by blood to different cells. There it gets incorporated, by an as yet unknown mechanism, into target proteins.. A lengthier article on the system of human iron regulation can be found in the article on human iron metabolism.
Iron in organic synthesis
The usage of iron metal filings in organic synthesis is mainly for the reduction of nitro compounds. Additionally, iron has been used for desulfurizations, reduction of aldehydes, and the deoxygenation of amine oxides.
Precautions
Excessive iron is toxic to humans, because excess ferrous iron reacts with peroxides in the body, producing free radicals. Iron becomes toxic when it exceeds the amount of transferrin needed to bind free iron. In excess, uncontrollable quantities of free radicals are produced.
Iron uptake is tightly regulated by the human body, which has no physiologic means of excreting iron and regulates iron solely by regulating uptake. However, too much ingested iron can damage the cells of the gastrointestinal tract directly, and may enter the bloodstream by damaging the cells that would otherwise regulate its entry. Once there, it causes damage to cells in the heart, liver and elsewhere. This can cause serious problems, including the potential of death from overdose, and long-term organ damage in survivors.
Humans experience iron toxicity above 20 milligrams of iron for every kilogram of weight, and 60 milligrams per kilogram is a lethal dose. Over-consumption of iron, often the result of children eating large quantitities of ferrous sulfate tablets intended for adult consumption, is the most common toxicological cause of death in children under six. The DRI lists the Tolerable Upper Intake Level (UL) for adults as 45 mg/day. For children under fourteen years old the UL is 40 mg/day.
If iron intake is excessive iron overload disorders can sometimes result, such as hemochromatosis. Iron overload disorders require a genetic inability to regulate iron uptake; however, many people have a genetic susceptibility to iron overload without realizing it and without knowing a family history of the problem. For this reason, people should not take iron supplements unless they suffer from iron deficiency and have consulted a doctor. Blood donors are at special risk of low iron levels and are often recommended to supplement their iron intake.
The medical management of iron toxicity is complex. One element of the medical approach is a specific chelating agent called deferoxamine, used to bind and expel excess iron from the body in case of iron toxicity.