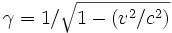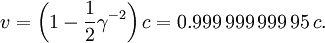Electron
2007 Schools Wikipedia Selection. Related subjects: Electricity and Electronics
| Electron | |
 Theoretical estimates of the electron density for the first few hydrogen atom electron orbitals shown as cross-sections with colour-coded probability density |
|
| Composition: | Elementary particle |
|---|---|
| Family: | Fermion |
| Group: | Lepton |
| Generation: | First |
| Interaction: | Gravity, Electromagnetic, Weak |
| Antiparticle: | Positron |
| Theorized: | G. Johnstone Stoney ( 1874) |
| Discovered: | J.J. Thomson ( 1897) |
| Mass: | 9.109 3826(16) × 10–31 kg 5.485 799 0945(24) × 10–4 u 1⁄1822.888 4849(8) u |
| Electric charge: | –1.602 176 53(14) × 10–19 C |
| Spin: | ½ |
The Electron is a fundamental subatomic particle that carries an electric charge. It is a spin-½ lepton that participates in electromagnetic interactions, and its mass is less than one thousandth of that of the smallest atom. Its electric charge is defined by convention to be negative, with a value of −1 in atomic units. Together with atomic nuclei, electrons make up atoms; their interaction with adjacent nuclei is the main cause of chemical bonding.
Overview
The word electron was coined in 1891 by George Johnstone Stoney and is derived from the term electric force introduced by William Gilbert. Its origin is in Greek: ήλεκτρον (elektron), meaning amber. J.J. Thomson is credited with having first measured the charge/mass ratio and is considered to be the discoverer of the electron.
Within an atom, electrons surround a nucleus composed of protons and neutrons in an electron configuration. The variations in electric field generated by differing numbers of electrons and their configurations in atoms determine the chemical properties of the elements. These fields play a fundamental role in chemical bonds and chemistry.
Electrons in motion produce an electric current and a magnetic field. Some types of electric currents are termed electricity.
Our understanding of how electrons behave has been significantly modified during the past century, the greatest advances being the development of quantum mechanics in the 20th century. This brought the idea of wave-particle duality, that is, that electrons show both wave-like and particle-like properties, to varying degrees. Equally important, particle physics has furthered our understanding of how the electron interacts with other particles.
Classification
The electron is one of a class of subatomic particles called leptons, which are believed to be fundamental particles (that is, they cannot be broken down into smaller constituent parts).
As with all particles, electrons can also act as waves. This is called the wave-particle duality, also known by the term complementarity coined by Niels Bohr and can be demonstrated using the double-slit experiment.
The antiparticle of an electron is the positron, which has the same mass but positive rather than negative charge. The discoverer of the positron, Carl D. Anderson, proposed calling standard electrons negatrons, and using electron as a generic term to describe both the positively and negatively charged variants. This usage never caught on and is rarely if ever encountered today.
Properties and behaviour
Electrons have a negative electric charge of −1.6022 × 10−19 coulomb, a mass of 9.11 × 10−31 kg based on charge/mass measurements and a relativistic rest mass of about 0.511 MeV/c2. The mass of the electron is approximately 1/1836 of the mass of the proton. The common electron symbol is e−.
According to quantum mechanics, electrons can be represented by wavefunctions, from which a calculated probabilistic electron density can be determined. The orbital of each electron in an atom can be described by a wavefunction. Based on the Heisenberg uncertainty principle, the exact momentum and position of the actual electron cannot be simultaneously determined. This is a limitation which, in this instance, simply states that the more accurately we know a particle's position, the less accurately we can know its momentum, and vice versa.
The electron has spin ½ and is a fermion (it follows Fermi-Dirac statistics). In addition to its intrinsic angular momentum, an electron has an intrinsic magnetic moment along its spin axis.
Electrons in an atom are bound to that atom; electrons moving freely in vacuum, space or certain media are free electrons that can be focused into an electron beam. When free electrons move, there is a net flow of charge, this flow is called an electric current. The drift velocity of electrons in metal wires is on the order of mm/hour. However, the speed at which a current at one point in a wire causes a current in other parts of the wire is typically 75% of light speed.
In some superconductors, pairs of electrons move as Cooper pairs in which their motion is coupled to nearby matter via lattice vibrations called phonons. The distance of separation between Cooper pairs is roughly 100 nm. (Rohlf, J.W.)
A body has an electric charge when that body has more or fewer electrons than are required to balance the positive charge of the nuclei. When there is an excess of electrons, the object is said to be negatively charged. When there are fewer electrons than protons, the object is said to be positively charged. When the number of electrons and the number of protons are equal, their charges cancel each other and the object is said to be electrically neutral. A macroscopic body can develop an electric charge through rubbing, by the phenomenon of triboelectricity.
When electrons and positrons collide, they annihilate each other and produce pairs of high energy photons or other particles. On the other hand, high-energy photons may transform into an electron and a positron by a process called pair production, but only in the presence of a nearby charged particle, such as a nucleus.
The electron is currently described as a fundamental particle or an elementary particle. It has no substructure (although British physicist Humphrey Maris claims to have found a way to split the electron into "electrinos" using an electron bubble). Hence, for convenience, it is usually defined or assumed to be a point-like mathematical point charge, with no spatial extension. However, when a test particle is forced to approach an electron, we measure changes in its properties (charge and mass). This effect is common to all elementary particles: Current theory suggests that this effect is due to the influence of vacuum fluctuations in its local space, so that the properties measured from a significant distance are considered to be the sum of the bare properties and the vacuum effects (see renormalization).
The classical electron radius is 2.8179 × 10−15 m. This is the radius that is inferred from the electron's electric charge, by using the classical theory of electrodynamics alone, ignoring quantum mechanics. Classical electrodynamics ( Maxwell's electrodynamics) is the older concept that is widely used for practical applications of electricity, electrical engineering, semiconductor physics, and electromagnetics; quantum electrodynamics, on the other hand, is useful for applications involving modern particle physics and some aspects of optical, laser and quantum physics.
Based on current theory, the speed of an electron can approach, but never reach, c (the speed of light in a vacuum). This limitation is attributed to Einstein's theory of special relativity which defines the speed of light as a constant within all inertial frames. However, when relativistic electrons are injected into a dielectric medium, such as water, where the local speed of light is significantly less than c, the electrons will (temporarily) be traveling faster than light in the medium. As they interact with the medium, they generate a faint bluish light, called Cherenkov radiation.
The effects of special relativity are based on a quantity known as γ or the Lorentz factor. γ is a function of v, the velocity of the particle, and c. It is defined as:
The energy necessary to accelerate a particle is γ minus one times the rest mass. For example, the linear accelerator at Stanford can accelerate an electron to roughly 51 GeV . This gives a gamma of 100,000, since the rest mass of an electron is 0.51 MeV/c² (the relativistic mass of this electron is 100,000 times its rest mass). Solving the equation above for the speed of the electron (and using an approximation for large γ) gives:
In practice
In the universe
Scientists believe that the number of electrons existing in the known universe is at least 1079. This number amounts to an average density of about one electron per cubic metre of space. Astronomers have determined that 90% of all of the detectable mass in the universe is hydrogen, which is made of one electron and one proton.
Based on the classical electron radius and assuming a dense sphere packing, it can be calculated that the number of electrons that would fit in the observable universe is on the order of 10130.
In industry
Electron beams are used in welding, lithography, scanning electron microscopes and transmission electron microscopes. LEED and RHEED are also important tools where electrons are used.
They are also at the heart of cathode ray tubes, which are used extensively as display devices in laboratory instruments, computer monitors and television sets. In photomultiplier tubes, one photon strikes the photocathode, initiating an avalanche of electrons that produces a detectable current.
In the laboratory
Electron microscopes are used to magnify details up to 500,000 times. Quantum effects of electrons are used in Scanning tunneling microscope to study features at the atomic scale.
In theory
In relativistic quantum mechanics, the electron can be described by the Dirac Equation which defines the electron as a (mathematical) point. In quantum field theory, the behaviour of the electron can be described by quantum electrodynamics (QED), a U(1) gauge theory. In Dirac's model, an electron is defined to be a mathematical point, a point-like, charged "bare" particle surrounded by a sea of interacting pairs of virtual particles and antiparticles . These provide a correction of just over 0.1% to the predicted value of the electron's gyromagnetic ratio from exactly 2 (as predicted by Dirac's single-particle model). The extraordinarily precise agreement of this prediction with the experimentally determined value is viewed as one of the great achievements of modern physics.
In the Standard Model of particle physics, the electron is the first- generation charged lepton. It forms a weak isospin doublet with the electron neutrino; these two particles interact with each other through the both the charged and neutral current weak interaction. The electron is very similar to the two more massive particles of higher generations, the muon and the tau lepton, which are identical in charge, spin, interaction but differ in mass.
The antimatter counterpart of the electron is the positron. The positron has the same amount of electrical charge as the electron, except that the charge is positive. It has the same mass and spin as the electron. When an electron and a positron meet, they may annihilate each other, giving rise to two gamma-ray photons. If the electron and positron had negligible momentum, each gamma ray will have an energy of 0.511 MeV. See also Electron-positron annihilation.
Electrons are a key element in electromagnetism, a theory that is accurate for macroscopic systems, and for classical modelling of microscopic systems.
History
The electron as a unit of charge in electrochemistry was posited by G. Johnstone Stoney in 1874, who also coined the term electron in 1894. During the late 1890s a number of physicists posited that electricity could be conceived of as being made of discrete units, which were given a variety of names, but their reality had not been confirmed in a compelling way.
The discovery that the electron was a subatomic particle was made in 1897 by J.J. Thomson at the Cavendish Laboratory at Cambridge University, while he was studying cathode ray tubes. A cathode ray tube is a sealed glass cylinder in which two electrodes are separated by a vacuum. When a voltage is applied across the electrodes, cathode rays are generated, causing the tube to glow. Through experimentation, Thomson discovered that the negative charge could not be separated from the rays (by the application of magnetism), and that the rays could be deflected by an electric field. He concluded that these rays, rather than being waves, were composed of negatively charged particles he called "corpuscles". He measured their mass-to-charge ratio and found it to be over a thousand times smaller than that of a hydrogen ion, suggesting that they were either very highly charged or very small in mass. Later experiments by other scientists upheld the latter conclusion.
The electron's charge was carefully measured by Robert Millikan in his oil-drop experiment of 1909.
The periodic law states that the chemical properties of elements largely repeat themselves periodically and is the foundation of the periodic table of elements. The law itself was initially explained by the atomic mass of the elements. However, as there were anomalies in the periodic table, efforts were made to find a better explanation for it. In 1913, Henry Moseley introduced the concept of the atomic number and explained the periodic law in terms of the number of protons each element has. In the same year, Niels Bohr showed that electrons are the actual foundation of the table. In 1916, Gilbert Newton Lewis explained the chemical bonding of elements by electronic interactions.
|
Quantum electrodynamics
|
|
|
electron | positron | photon |
|