Phase (matter)
2007 Schools Wikipedia Selection. Related subjects: General Physics
In the physical sciences, a phase is a set of states of a macroscopic physical system that have relatively uniform chemical composition and physical properties (i.e. density, crystal structure, index of refraction, and so forth). The most familiar examples of phases are solids, liquids, and gases. Less familiar phases include: plasmas and quark-gluon plasmas; Bose-Einstein condensates and fermionic condensates; strange matter; liquid crystals; superfluids and supersolids; and the paramagnetic and ferromagnetic phases of magnetic materials.
Phases are sometimes called states of matter, but this term can lead to confusion with thermodynamic states. For example, two gases maintained at different pressures are in different thermodynamic states, but the same "state of matter".
General definition of phases
In general, we say that two different states of a system are in different phases if there is an abrupt change in their physical properties while transforming from one state to the other. Conversely, two states are in the same phase if they can be transformed into one another without any abrupt changes.
An important point is that different types of phases are associated with different physical qualities. When discussing the solid, liquid, and gaseous phases, we talked about rigidity and compressibility, and the effects of varying the pressure and volume, because those are the relevant properties that distinguish a solid, a liquid, and a gas. On the other hand, when discussing paramagnetism and ferromagnetism, we looked at the magnetization, because that is what distinguishes the ferromagnetic phase from the paramagnetic phase. Several more examples of phases will be given in the following section.
Not all physical quantities are relevant when we are looking at a certain system. For example, it is generally not useful for us to compare the magnetization of liquid water to the magnetization of ice. In this sense, what constitutes a "phase" depends on what parameters you are looking at, and vice versa. It is this idea that allows us to generalize the concept of phases to encompass a wide variety of phenomena.
In more technical language, a phase is a region in the parameter space of thermodynamic variables in which the free energy is analytic. As long as the free energy is analytic, all thermodynamic properties (such as entropy, heat capacity, magnetization, and compressibility) will be well-behaved, because they can be expressed in terms of the free energy and its derivatives. For example, the entropy is the first derivative of the free energy with temperature.
When a system goes from one phase to another, there will generally be a stage where the free energy is non-analytic. This is a phase transition. Due to this non-analyticity, the free energies on either side of the transition are two different functions, so one or more thermodynamic properties will behave very differently after the transition. The property most commonly examined in this context is the heat capacity. During a transition, the heat capacity may become infinite, jump abruptly to a different value, or exhibit a "kink" or discontinuity in its derivative. See also differential scanning calorimetry.
Other examples of phases
In this section, we will present several systems that exhibit phase phenomena.
We have discussed the solid, liquid, and gaseous phases of ordinary matter. It turns out that other configurations of molecules are possible, corresponding to novel phases. Amorphous solids, or glasses, are a solid phase in terms of mechanical behaviour, but lack the structural order of an ordinary ("crystalline") solid, so that the arrangement of atoms within them resembles a liquid. Liquid crystals are another phase intermediate between solids and liquids; the molecules of such a substance have an orderly orientation and in some cases (that is, for smectic liquid crystals) even an orderly position in one direction, but are free to flow past one another. Liquid crystals are liquids in the mechanical sense, but have structural features that are normally seen in solids.
In many materials, there are actually a variety of solid phases, each corresponding to a unique crystal structure. These varying crystal phases of the same substance are called " allotropes" if intramolecular bonding changes or "polymorphs" if only intermolecular bonding changes. For instance, there are at least nine different polymorphs of ice that manifest under different temperature and pressure conditions. To take another example, diamond and graphite are allotropes of carbon. Graphite is composed of layers of hexagonally arranged carbon atoms, in which each carbon atom is strongly bound to three neighboring atoms in the same layer and is weakly bound to atoms in the neighboring layers. By contrast, in diamond each carbon atom is strongly bound to four neighboring carbon atoms in a diamond cubic array, with tetrahedral bonding. The unique crystal structures of graphite and diamond are responsible for the vastly different properties of these two materials.
In an ordinary gas phase, the electrons are tightly bound to the atomic nuclei. In contrast, in the plasma phase the atoms are dissociated, i.e. the electrons are separated from the atomic nuclei. This dissociation, or ionization, occurs abruptly upon raising the temperature and lowering the pressure, and thus displays the hallmarks of a phase transition.
Bose-Einstein condensate is a phase of matter that occurs at extremely low temperatures, near absolute zero. These temperatures are too low to occur anywhere on Earth except in laboratory experiments. The very slow motion of molecules at these temperatures allow some of the more bizarre aspects of quantum mechanics to manifest themselves in the form of novel macroscopic properties.
Phases can also exist in two dimensions. The boundaries between two different three-dimensional phases, the surfaces of materials, and the grain boundaries between different crystallographic orientations of a single material can also show distinct phases. For example, surface reconstructions on metal and semiconductor surfaces are two dimensional phases.
Under extremely high pressure, ordinary matter undergoes a transition to a series of exotic phases collectively known as degenerate matter. These phases are of great interest to astrophysics, because these high-pressure conditions are believed to exist inside stars that have used up their nuclear fusion "fuel", such as white dwarves and neutron stars.
Phase transitions also play an extremely important role in physical cosmology. It is believed that the universe as a whole underwent a series of important phase transitions during its early history, shortly after the Big Bang. A major branch of theoretical cosmology, inflation theory, seeks to explain various aspects of the modern universe, such as why the universe is so flat, as the effect of one or more of these transitions. These transitions are of great interest to particle physics as well, as it has been hypothesized that the quantum field that fills spacetime (a particle physics concept that incorporates "material" particles like electrons as well as "field-like" particles such as photons and gluons) underwent a series of transitions from a highly "symmetric" phase in which all fundamental forces were unified into a single entity, into the " broken symmetry" phase that we observe today, in which there are four fundamental forces with very different strengths.
Phase diagrams
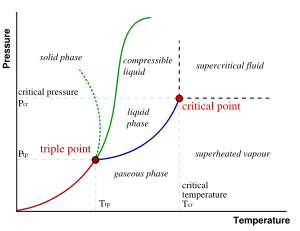
The different phases of a system may be represented using a phase diagram. The axes of the diagrams are the relevant thermodynamic variables. For simple mechanical systems, we generally use the pressure and temperature.
The markings on the phase diagram show the points where the free energy is non-analytic. The open spaces, where the free energy is analytic, correspond to the phases. The phases are separated by lines of non-analyticity, where phase transitions occur, which are called phase boundaries.
In the diagram, the phase boundary between liquid and gas does not continue indefinitely. Instead, it terminates at a point on the phase diagram called the critical point. At temperatures and pressure above the critical point, the physical property differences that differentiate the liquid phase from the gas phase become less defined. This reflects the fact that, at extremely high temperatures and pressures, the liquid and gaseous phases become indistinguishable. In water, the critical point occurs at around 647 K (374 °C or 705 °F) and 22.064 MPa.
The existence of the liquid-gas critical point reveals a slight ambiguity in our above definitions. When going from the liquid to the gaseous phase, one usually crosses the phase boundary, but it is possible to choose a path that never crosses the boundary by going to the right of the critical point. Thus, phases can sometimes blend continuously into each other. This new phase which has some properties that are similar to a liquid and some properties that are similar to a gas is called a supercritical fluid. We should note, however, that this does not always happen. For example, it is impossible for the solid-liquid phase boundary to end in a critical point in the same way as the liquid-gas boundary, because the solid and liquid phases have different symmetry.
An interesting thing to note is that the solid-liquid phase boundary in the phase diagram of most substances, such as the one shown above, has a positive slope. This is due to the solid phase having a higher density than the liquid, so that increasing the pressure increases the melting temperature. However, in the phase diagram for water the solid-liquid phase boundary has a negative slope. This reflects the fact that ice has a lower density than water, which is an unusual property for a material.
Metastable phases
Sometimes a substance or mixture can be heated, compressed, etc., beyond the point at which it would normally exhibit a phase change, but without actually triggering the change. Examples include supercooling, superheating, and supersaturation.
Usually each polymorph of a given substance is only stable over a specific range of conditions. For example, diamond is the stable form of carbon at extremely high pressures while graphite is the stable form at normal atmospheric pressures. Regardless, diamonds appear stable at normal temperatures and pressures, but, in fact, are very slowly converting to graphite. Heat increases the rate of this transformation, but at normal temperatures the diamond is practically stable.
Another important example of metastable polymorphs occurs in the processing of steel. Steels are often subjected to a variety of thermal treatments designed to produce various combinations of stable and metastable iron phases. In this way the steel properties, such as hardness and strength can be adjusted by controlling the relative amounts and crystal sizes of the various phases that form.
Phase equilibrium
The distribution of kinetic energy among molecules is not uniform, and it changes randomly. This means that at, say, the surface of a liquid, there may be an individual molecule with enough kinetic energy to jump into the gas phase. Likewise, individual gas molecules may have low enough kinetic energy to join other molecules in the liquid phase. This phenomenon means that at any given temperature and pressure, multiple phases may co-exist.
For example, under standard conditions for temperature and pressure, a bowl of liquid water in dry air will evaporate until the partial pressure of gaseous water equals the vapor pressure of water. At this point, the rate of molecules leaving and entering the liquid phase becomes the same (due to the increased number of gaseous water molecules available to re-condense). The fact that liquid molecules with above-average kinetic energy have been removed from the bowl results in evaporative cooling. Similar processes may occur on other types of phase boundaries.
Gibbs' phase rule relates the number of possible phases, variables such as temperature and pressure, and whether or not an equilibrium will be reached.
Emergence and universality
Phases are emergent phenomena produced by the self-organization of a macroscopic number of particles. Typical samples of matter, for example, contain around 1023 particles (of the order of Avogadro's number). In systems that are too small -- even, say, a thousand atoms -- the distinction between phases disappears, since the appearance of non-analyticity in the free energy requires a huge, formally infinite, number of particles to be present.
One might ask why real systems exhibit phases, since they are not actually infinite. The reason is that real systems contain thermodynamic fluctuations. When a system is far from a phase transition, these fluctuations are unimportant, but as it approaches a phase transition, the fluctuations begin to grow in size (i.e. spatial extent). At the ideal transition point, their size would be infinite, but before that can happen the fluctuations will have become as large as the system itself. In this regime, "finite-size" effects come into play, and we are unable to accurately predict the behaviour of the system. Thus, phases in a real system are only well-defined away from phase transitions, and how far away it needs to be is dependent on the size of the system.
There is a corollary to the emergent nature of phase phenomena, known as the principle of universality. The properties of phases are largely independent of the underlying microscopic physics, so that the same types of phases arise in a wide variety of systems. This is a familiar fact of life. We know, for example, that the property that defines a solid -- resistance to deformation -- is exhibited by materials as diverse as iron, ice, and Silly Putty. The only differences are matters of scale. Iron may resist deformation more strongly than Silly Putty, but both maintain their shape if the applied forces are not too strong.