Copper
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||
| General | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
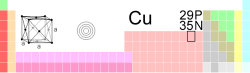
| Name, Symbol, Number | copper, Cu, 29 | ||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||
| Group, Period, Block | 11, 4, d | ||||||||||||||||||
| Appearance | metallic pinkish red |
||||||||||||||||||
| Atomic mass | 63.546 (3) g/mol | ||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s1 | ||||||||||||||||||
| Electrons per shell | 2, 8, 18, 1 | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Phase | solid | ||||||||||||||||||
| Density (near r.t.) | 8.96 g·cm−3 | ||||||||||||||||||
| Liquid density at m.p. | 8.02 g·cm−3 | ||||||||||||||||||
| Melting point | 1357.77 K (1084.62 ° C, 1984.32 ° F) |
||||||||||||||||||
| Boiling point | 2835 K (2562 ° C, 4643 ° F) |
||||||||||||||||||
| Heat of fusion | 13.26 kJ·mol−1 | ||||||||||||||||||
| Heat of vaporization | 300.4 kJ·mol−1 | ||||||||||||||||||
| Heat capacity | (25 °C) 24.440 J·mol−1·K−1 | ||||||||||||||||||
|
|||||||||||||||||||
| Atomic properties | |||||||||||||||||||
| Crystal structure | face centered cubic | ||||||||||||||||||
| Oxidation states | 2, 1 (mildly basic oxide) |
||||||||||||||||||
| Electronegativity | 1.90 (Pauling scale) | ||||||||||||||||||
| Ionization energies ( more) |
1st: 745.5 kJ·mol−1 | ||||||||||||||||||
| 2nd: 1957.9 kJ·mol−1 | |||||||||||||||||||
| 3rd: 3555 kJ·mol−1 | |||||||||||||||||||
| Atomic radius | 135 pm | ||||||||||||||||||
| Atomic radius (calc.) | 145 pm | ||||||||||||||||||
| Covalent radius | 138 pm | ||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||
| Miscellaneous | |||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||
| Electrical resistivity | (20 °C) 16.78 nΩ·m | ||||||||||||||||||
| Thermal conductivity | (300 K) 401 W·m−1·K−1 | ||||||||||||||||||
| Thermal expansion | (25 °C) 16.5 µm·m−1·K−1 | ||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) (annealed) 3810 m·s−1 |
||||||||||||||||||
| Young's modulus | 130 GPa | ||||||||||||||||||
| Shear modulus | 48 GPa | ||||||||||||||||||
| Bulk modulus | 140 GPa | ||||||||||||||||||
| Poisson ratio | 0.34 | ||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||
| Vickers hardness | 369 MPa | ||||||||||||||||||
| Brinell hardness | 874 MPa | ||||||||||||||||||
| CAS registry number | 7440-50-8 | ||||||||||||||||||
| Selected isotopes | |||||||||||||||||||
|
|||||||||||||||||||
| References | |||||||||||||||||||
Copper ( IPA: /ˈkɒpə/) is a chemical element in the periodic table that has the symbol Cu ( Latin: cuprum) and atomic number 29. It is a ductile metal with excellent electrical conductivity, and finds extensive use as an electrical conductor, thermal conductor, as a building material, and as a component of various alloys.
Copper is an essential nutrient to all higher plants and animals. In animals, it is found primarily in the bloodstream, as a cofactor in various enzymes, and in copper-based pigments. In sufficent amounts, copper can be poisonous or even fatal to organisms.
Copper has played a significant part in the history of mankind, which has used the easily accessible uncompounded metal for nearly 10,000 years. Civilizations in places like Iraq, China, Egypt, Greece and the Sumerian cities all have early evidence of using copper, and Britain and the United States also have extensive histories of copper use and mining. During the Roman Empire, copper was principally mined on Cyprus, hence the origin of the name of the metal as Cyprium, "metal of Cyprus", later shortened to Cuprum. A number of countries, such as Chile and the United States, still have sizeable reserves of the metal which are extracted through large open mines. Nevertheless, the price of copper rose rapidly—quintupling from a 60-year low in 1999—largely due to increased demand. As of mid-2006, the price has stabilized.
Notable characteristics
Copper is a reddish-colored metal, with a high electrical and thermal conductivity (silver is the only pure metal to have a higher electrical conductivity at room temperature). In oxidation copper is mildly basic. Copper has its characteristic colour because it reflects red and orange light and absorbs other frequencies in the visible spectrum, due to its band structure. This can be contrasted with the optical properties of silver, gold and aluminium.
Copper occupies the same family of the periodic table as silver and gold, because it shares many characteristics with these metals. All have very high thermal and electrical conductivity, and all are malleable metals.
In its liquid state, a clear copper surface without ambient light appears somewhat greenish, another characteristic shared with gold. Silver does not have this property, so it is not a complementary color for the orange incandescence colour. When liquid copper is in bright ambient light, it retains some of its pinkish luster. Due to its high surface tension, the liquid metal does not wetten surfaces but instead forms spherical droplets when poured on a surface.
Copper is insoluble in water (H2O) as well as in isopropanol, or isopropyl alcohol.
There are two stable isotopes, 63Cu and 65Cu, along with a couple dozen radioisotopes. The vast majority of radioisotopes have half lives on the order of minutes or less; the longest lived, 64Cu, has a half life of 12.7 hours, with two decay modes leading to two separate products.
Numerous alloys of copper exist, many with important historical and contemporary uses. Speculum metal and bronze are alloys of copper and tin. Brass is an alloy of copper and zinc. Monel metal, also called cupronickel, is an alloy of copper and nickel. While the metal "bronze" usually refers to copper-tin alloys, it also is a generic term for any alloy of copper, such as aluminium bronze, silicon bronze, and manganese bronze.
The purity of copper is expressed as 4N for 99.99% pure or 7N for 99.99999% pure. The numeral gives the number of nines after the decimal point when expressed as a decimal (eg 4N means 0.9999, or 99.99%).
Applications
Copper is malleable and ductile, a good conductor of heat and, when very pure, a good conductor of electricity.
It is used extensively, in products such as:
- Electronics:
- Copper wire.
- Electromagnets.
- Electrical machines, especially electromagnetic motors and generators.
- Electrical relays, electrical busbars and electrical switches.
- Vacuum tubes, cathode ray tubes, and the magnetrons in microwave ovens.
- Wave guides for microwave radiation.
- Integrated circuits, increasingly replacing aluminium because of its superior electrical conductivity.
- As a material in the manufacture of computer heat sinks, as a result of its superior heat dissipation capacity to aluminium.
- Structural Engineering:
- Statuary: The Statue of Liberty, for example, contains 179,200 pounds (81.3 tonnes) of copper.
- Alloyed with nickel, e.g. cupronickel and Monel, used as corrosive resistant materials in shipbuilding.
- Watt's steam engine.
- Household Products:
- Copper plumbing fittings and compression tubes.
- Doorknobs and other fixtures in houses.
- Roofing, guttering, and rainspouts on buildings.
- In cookware, such as frying pans.
- Most flatware ( knives, forks, spoons) contains some copper ( nickel silver).
- Sterling silver, if it is to be used in dinnerware, must contain a few percent copper.
- Copper was sometimes used by the Inuit to make the cutting blade for ulus.
- Copper water heating cylinders
- Coinage:
- Biomedical applications:
- As a biostatic surface in hospitals, and to line parts of ships to protect against barnacles and mussels, originally used pure, but superseded by Muntz Metal. Bacteria will not grow on a copper surface because it is biostatic. Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaire's Disease is suppressed by copper tubing in air-conditioning systems.
- Copper(II) sulfate is used as a fungicide and as algae control in domestic lakes and ponds. It is used in gardening powders and sprays to kill mildew.
- Copper-62-PTSM, a complex contaning radioactive copper-62, is used as a Positron emission tomography radiotracer for heart blood flow measurements.
- Chemical applications:
- Compounds, such as Fehling's solution, have applications in chemistry.
- As a component in ceramic glazes, and to colour glass.
- Catalysis:
- Used in the Water gas shift reaction which converts carbon monoxide into carbon dioxide.
- Steam reforming which extracts hydrogen from hydrocarbons.
- Others:
- Musical instruments, especially brass instruments and cymbals.
History
The Egyptians found that adding a small amount of tin made the metal easier to cast, so bronze alloys were found in Egypt almost as soon as copper was found. Use of copper in ancient China dates to at least 2000 BC. By 1200 BC excellent bronzes were being made in China. Note that these dates are affected by wars and conquest, as copper is easily melted down and reused. In Europe, Oetzi the Iceman, a well-preserved male dated to 3200 BC, was found with a copper-tipped axe whose metal was 99.7% pure. High levels of arsenic in his hair suggests he was involved in copper smelting. Brass, an alloy of zinc and copper, was known to the Greeks but first used extensively by the Romans.
There are copper and bronze artifacts from Sumerian cities that date to 3000 BC, and Egyptian artifacts in copper and copper alloyed with tin nearly as old. In one pyramid, a copper plumbing system was found that is 5000 years old.
In Greek times, the metal was known by the name chalkos (χαλκός). Copper was a very important resource for the Romans and Greeks. In Roman times, it became known as aes Cyprium (aes being the generic Latin term for copper alloys such as bronze and other metals, and Cyprium because so much of it was mined in Cyprus). From this, the phrase was simplified to cuprum and then eventually Anglicized into the English copper. Copper was associated with the goddess Aphrodite/ Venus in mythology and alchemy, owing to its lustrous beauty, its ancient use in producing mirrors, and its association with Cyprus, which was sacred to the goddess. In alchemy the symbol for copper was also the symbol for the planet Venus.
Copper, as native copper, is one of the few metals to naturally occur as an uncompounded mineral. Copper was known to some of the oldest civilizations on record, and has a history of use that is at least 10,000 years old. A copper pendant was found in what is now northern Iraq that dates to 8700 BC. By 5000 BC, there are signs of copper smelting, the refining of copper from simple copper compounds such as malachite or azurite. Among archaeological sites in Anatolia, Çatal Höyük (~6000 BC) features native copper artifacts and smelted lead beads, but no smelted copper. But Can Hasan (~5000 BC) had access to smelted copper; this site has yielded the oldest known cast copper artifact, a copper mace head.
Copper smelting appears to have been developed independently in several parts of the world. In addition to its development in Anatolia by 5000 BC, it was developed in China before 2800 BC, in Central America around 600 AD, and in West Africa around 900 AD.
The use of bronze was so pervasive in a certain era of civilization that it has been named the Bronze Age. The transitional period in certain regions between the preceding Neolithic period and the Bronze Age is termed the Chalcolithic, with some high-purity copper tools being used alongside stone tools.
Copper mining in Britain and the United States
Copper has been mined for many centuries. By 2000 BC, Europe was using copper-tin alloys or ‘bronze’. The Bronze Age is taken as 2500 BC to 600 BC.
British Isles
During the Bronze age, copper was mined in the British Isles mainly in the following locations:
- South West County Cork
- West Wales (e.g. Cwmwystwyth)
- North Wales (e.g. Great Orme)
- Anglesey (Parys Mountain)
- Cheshire ( Alderley Edge)
- The Staffordshire Moorlands (e.g. Ecton Mine)
- Isle of Man, which is between England and Northern Ireland
At Great Orme in North Wales, such working extended for a depth of 70 metres. At Alderley Edge in Cheshire, carbon dates have established mining at around 2280 - 1890 BC (at 95% probability).
United States
Copper mining in United States began with marginal workings by Native Americans and some development by early Spaniards. Europeans were mining copper in Connecticut as early as 1709. Westward movement also brought an expansion of copper exploitation with developments of significant deposits in Michigan and Arizona during the 1850's and then in Montana during the 1860's.
Copper was mined extensively in Michigan's Keweenaw Peninsula with the heart of extraction at the productive Quincy Mine. Arizona had many notable deposits including the Copper Queen in Bisbee and the United Verde in Jerome. The Anaconda in Butte, Montana became the nation's chief copper supplier by 1886.
Copper has also been made in Utah, Nevada and Tennessee, and among other locations.
Biological role
Copper is essential in all higher plants and animals. Copper is carried mostly in the bloodstream on a plasma protein called ceruloplasmin. When copper is first absorbed in the gut it is transported to the liver bound to albumin. Copper is found in a variety of enzymes, including the copper centers of cytochrome c oxidase and the enzyme superoxide dismutase (containing copper and zinc). In addition to its enzymatic roles, copper is used for biological electron transport. The blue copper proteins that participate in electron transport include azurin and plastocyanin. The name "blue copper" comes from their intense blue colour arising from a ligand-to-metal charge transfer (LMCT) absorption band around 600 nm.
Most molluscs and some arthropods such as the horseshoe crab use the copper-containing pigment hemocyanin rather than iron-containing hemoglobin for oxygen transport, so their blood is blue when oxygenated rather than red.
It is believed that zinc and copper compete for absorption in the digestive tract so that a diet that is excessive in one of these minerals may result in a deficiency in the other. The RDA for copper in normal healthy adults is 0.9 mg/day.
Toxicity
All copper compounds, unless otherwise known, should be treated as if they were toxic. Thirty grams of copper sulfate is potentially lethal in humans. The suggested safe level of copper in drinking water for humans varies depending on the source, but tends to be pegged at 1.5 to 2 mg/L. The DRI Tolerable Upper Intake Level for adults of dietary copper from all sources is 10 mg/day. In toxicity, copper can inhibit the enzyme dihydrophil hydratase, an enzyme involved in haemopoiesis.
A significant portion of the toxicity of copper comes from its ability to accept and donate single electrons as it changes oxidation state. This catalyzes the production of very reactive radical ions such as hydroxyl radical in a manner similar to fenton chemistry. This catalytic activity of copper is used by the enzymes that it is associated with and is thus only toxic when unsequestered and unmediated. This increase in unmediated reactive radicals is generally termed oxidative stress and is an active area of research in a variety of diseases where copper may play an important but more subtle role than in acute toxicity.
An inherited condition called Wilson's disease causes the body to retain copper, since it is not excreted by the liver into the bile. This disease, if untreated, can lead to brain and liver damage. In addition, studies have found that people with mental illnesses such as schizophrenia had heightened levels of copper in their systems. However it is unknown at this stage whether the copper contributes to the mental illness, whether the body attempts to store more copper in response to the illness, or whether the high levels of copper are the result of the mental illness.
Too much copper in water has also been found to damage marine life. The observed effect of these higher concentrations on fish and other creatures is damage to gills, liver, kidneys, and the nervous system.
Occurrence
The main copper ore producing countries are Chile, United States, Indonesia, Australia, Peru, Russia, Canada, China, Poland, Kazakhstan and Mexico.
Copper can be found as native copper in mineral form. Minerals such as the sulfides: chalcopyrite (CuFeS2), bornite (Cu5FeS4), covellite (CuS), chalcocite (Cu2S) are sources of copper, as are the carbonates: azurite (Cu3(CO3)2(OH)2) and malachite (Cu2CO3(OH)2) and the oxide: cuprite (Cu2O). Native copper also forms in uneconomic placer deposits.
Most copper ore is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0 percent copper. Examples include: Chuquicamata in Chile and El Chino Mine in New Mexico. The average abundance of copper found within crustal rocks is approximately 68 ppm by mass, and 22 ppm by atoms.
The Intergovernmental Council of Copper Exporting Countries (CIPEC), defunct since 1992, once tried to play a similar role for copper as OPEC does for oil, but never achieved the same influence, not least because the second-largest producer, the United States, was never a member. Formed in 1967, its principal members were Chile, Peru, Zaire, and Zambia.
The copper price has quintupled since 1999, rising from $0.60 per pound in June 1999 to $3.75 per pound in May 2006 .
Compounds
Common oxidation states of copper include the less stable copper(I) state, Cu+; and the more stable copper(II) state, Cu2+, which forms blue or blue-green salts and solutions. Under unusual conditions, a 3+ state and even an extremely rare 4+ state can be obtained.
Copper(II) carbonate is green from which arises the unique appearance of copper-clad roofs or domes on some buildings. Copper(II) sulfate forms a blue crystalline penta hydrate which is perhaps the most familiar copper compound in the laboratory. It is used as a fungicide, known as Bordeaux mixture.
There are two stable copper oxides, copper(II) oxide (CuO) and copper(I) oxide (Cu2O). Copper oxides are used to make yttrium barium copper oxide (YBa2Cu3O7-δ) or YBCO which forms the basis of many unconventional superconductors.
- Copper (I) compounds : copper(I) chloride, copper(I) bromide, copper(I) iodide, copper(I) oxide.
- Copper (II) compounds : copper(II) carbonate, copper(II) chloride, copper(II) hydroxide, copper(II) nitrate, copper(II) oxide, copper(II) sulfate, copper(II) sulfide.
- Copper (III) compounds , rare: potassium hexafluorocuprate (K3CuF6)
- Copper (IV) compounds , extremely rare: caesium hexafluorocuprate (Cs2CuF6)
Copper (I) and Copper (II) can also be referred to by their common names cuprous and cupric.
Tests for copper(II) ion
Add aqueous sodium hydroxide. A blue precipitate of copper(II) hydroxide should form, by the displacement of the copper ions by sodium ions.
Ionic equation:
- Cu2+(aq) + 2OH−(aq) → Cu(OH)2(s)
Add aqeuous ammonia. A precipitate should form, which then dissolves upon adding excess ammonia, to form a deep blue ammonia complex, tetraaminecopper(II).
Ionic equation:
- Cu2+(aq) + 4NH3(aq) → [Cu(NH3)4]2+(aq)