Copper(I) chloride
2007 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Copper(I) chloride | |
|---|---|
 |
|
| General | |
| Systematic name | Copper(I) chloride |
| Other names | Cuprous chloride |
| Molecular formula | CuCl |
| Molar mass | 98.99 g/mol |
| Appearance | white powder, slightly green from oxidation |
| CAS number | [7758-89-6] |
| Properties | |
| Density and phase | 4.140 g/cm3, solid |
| Solubility in water | 0.0062 g/100 ml (20 °C) |
| in ethanol | insoluble |
| in hydrochloric acid in diethyl ether in aqueous ammonia |
soluble |
| Melting point | 430 °C (703 K) |
| Boiling point | 1490 °C (1760 K), decomposes |
| Structure | |
| Crystal structure | Tetrahedral close packed ( Zinc blende structure) |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant |
| NFPA 704 | |
| Flash point | n/a |
| R/S statement | R: 22, 50, 53 S: 22, 60/61 |
| RTECS number | GL6990000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Copper(I) bromide Copper(I) iodide |
| Other cations | Copper(II) chloride Silver(I) chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references |
|
Copper(I) chloride (quite commonly called cuprous chloride), is the lower chloride of copper, with the formula CuCl. It occurs naturally as the mineral nantokite. It is a white solid which is almost insoluble in water, and which tends to oxidise in air to green CuCl2. It is a Lewis acid which reacts with suitable ligands such as ammonia or chloride ion to form complexes, many of which are water-soluble. It is even able to form a stable complex with carbon monoxide.
In aqueous solution, CuCl would be unstable with respect to disproportionation into Cu and CuCl2, but its low solubility allows it to be a stable compound.
Chemical Properties
Copper(I) chloride is a Lewis acid, classified as soft according to the Hard-Soft Acid-Base concept. Thus it tends to form stable complexes with soft Lewis bases such as triphenylphosphine:
CuCl + PPh3 → [CuCl(PPh3)]4 (Ph = phenyl)
Although CuCl is insoluble in water, it dissolves in aqueous solutions containing suitable donor molecules. It readily forms complexes with halide ions, for example forming H3O+ CuCl2- with concentrated hydrochloric acid. It also dissolves readily in solutions containing CN-, S2O32- or NH3
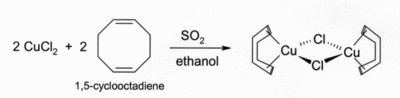
Solutions of CuCl in HCl or NH3 absorb carbon monoxide to form colourless complexes such as the crystalline halogen-bridged dimer [CuCl(CO)]2. The same HCl solution can also react with acetylene gas to form [CuCl(C2H2)], while an NH3 solution of CuCl forms an explosive acetylide with acetylene. Complexes of CuCl with alkenes can be made by reduction of CuCl2 by sulfur dioxide in the presence of the alkene in alcohol solution. Complexes with chelating alkenes such as 1,5-cyclooctadiene are particularly stable:
CuCl reacts with organometallic compounds such as methyllithium (CH3Li) to form "Gilman reagents" such as (CH3)2CuLi, which find extensive use in organic synthesis. Grignard reagents react similarly.
Preparation
Copper(I) chloride may be prepared by the reduction of copper(II) salts such as CuSO4 using sulfur dioxide or copper metal. SO2 may be prepared in situ from sodium bisulfite (NaHSO3) or sodium metabisulfite (Na2S2O5) and acid. The reduction is carried out in hydrochloric acid, and the resulting CuCl2- complex is diluted to precipitate white CuCl (by driving the equilibrium using Le Chatelier's principle).
(1) NaHSO3( aq) + HCl ( aq) → SO2( aq) + NaCl + H2O( l)
(2) 2 CuSO4( aq) + SO2( aq) + 2 H2O( l) + 4 HCl( aq) → 2 HCuCl2( aq) + 3 H2SO4( aq)
(3) HCuCl2( aq) + H2O( l) → CuCl( s) + H3O+( aq) + Cl-( aq)
Uses
A major chemical use for copper(I) chloride is as a catalyst for a variety of organic reactions. Compared to other "soft" Lewis acids, it is much more affordable than non-toxic silver(I) chloride and palladium(II) chloride, and much less toxic than lead(II) chloride and mercury(II) chloride. In addition, it can undergo redox chemistry via copper(II) or copper(III) intermediates. This combination of properties make copper(I) salts invaluable reagents.
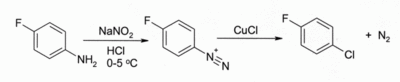
One such application is in the Sandmeyer reaction. Treatment of an arenediazonium salt with CuCl leads to an aryl chloride, for example:
The reaction has wide scope, and usually gives good yields.
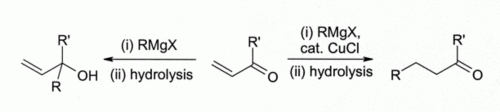
The observation that copper(I) halides catalyse 1,4-addition of Grignard reagents to alpha,beta-unsaturated ketones led to the development of organocuprate reagents that are widely used today in organic synthesis :
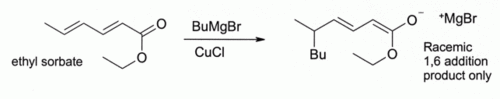
Although other copper(I) compounds such as copper(I) iodide are now more often used for this type of reaction, there are cases where copper(I) chloride is particularly effective:
Here, Bu indicates an n- butyl group. Without CuCl, the Grignard reagent alone gives a mixture of 1,2 and 1,4-addition products (i.e., the butyl adds at the closer to the C=O).
Copper(I) chloride is also an intermediate formed from copper(II) chloride in the Wacker process.
Precautions
Copper salts do have some toxicity and should be handled with care; wear gloves and goggles. Avoid bringing CuCl into contact with alkynes.
Template: inorganic stylesheet1