Uranium
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | uranium, U, 92 | ||||||||||||||||||||||||||||||||||||||||||
| Chemical series | actinides | ||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | n/a, 7, f | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery gray metallic; corrodes to a spalling black oxide coat in air  |
||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 238.02891 (3) g/mol | ||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f3 6d1 7s2 | ||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 21, 9, 2 | ||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 19.1 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 17.3 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1405.3 K (1132.2 ° C, 2070 ° F) |
||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4404 K (4131 ° C, 7468 ° F) |
||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 9.14 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 417.1 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 27.665 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic | ||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3+,4+,5+,6+ (weakly basic oxide) |
||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.38 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 597.6 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1420 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 175 pm | ||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 186 pm | ||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 0.280 µΩ·m | ||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 27.5 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 13.9 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 3155 m/s | ||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 208 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 111 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 100 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.23 | ||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-61-1 | ||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||
Uranium ( IPA: /jəˈreɪniəm/) is a chemical element in the periodic table that has the symbol U and atomic number 92. Heavy, silvery-white, metallic, naturally radioactive, uranium belongs to the actinide series. Its isotopes 235U and to a lesser degree 233U are used as the fuel for nuclear reactors and the explosive material for nuclear weapons. Depleted uranium ( 238U) is used in kinetic energy penetrators and armor plating.
Notable characteristics
When refined, uranium is a silvery white, weakly radioactive metal, which is slightly softer than steel. It is malleable, ductile, and slightly paramagnetic. Uranium metal has very high density, 65% more dense than lead, but slightly less dense than gold. When finely divided, it can react with cold water; in air, uranium metal becomes coated with a layer of uranium oxide. Uranium in ores is extracted chemically and converted into uranium dioxide or other chemical forms usable in industry.
Uranium metal has three allotropic forms:
- alpha (orthorhombic) stable up to 667.7 °C
- beta (tetragonal) stable from 667.7 °C to 774.8 °C
- gamma (body-centred cubic) from 774.8 °C to melting point - this is the most malleable and ductile state.
Natural uranium metal contains about 0.71% U-235, 99.28% U-238, and about 0.0054% U-234. In order to produce enriched uranium, the process of isotope separation removes a substantial portion of the U-235 for use in nuclear power, weapons, or other uses. The remainder, depleted uranium, contains only 0.2% to 0.4% U-235. Because natural uranium begins with such a low percentage of U-235, the enrichment process produces large quantities of depleted uranium. For example, producing 1 kg of 5% enriched uranium requires 11.8 kg of natural uranium, and leaves about 10.8 kg of depleted uranium with only 0.3% U-235 remaining.
Its two principal isotopes are 235U and 238U. Naturally-occurring uranium also contains a small amount of the 234U isotope, which is a decay product of 238U. The isotope 235U or enriched uranium is important for both nuclear reactors and nuclear weapons because it is the only isotope existing in nature to any appreciable extent that is fissile, that is, fissionable by thermal neutrons. The isotope 238U is also important because it absorbs neutrons to produce a radioactive isotope that subsequently decays to the isotope 239Pu (plutonium), which also is fissile.
The artificial 233U isotope is also fissile and is made from thorium-232 by neutron bombardment.
Uranium was the first element that was found to be fissile. Upon bombardment with slow neutrons, its 235U isotope becomes the very short lived 236U which immediately divides into two smaller nuclei, releasing nuclear binding energy and more neutrons. If these neutrons are absorbed by other 235U nuclei, a nuclear chain reaction occurs and, if there is nothing to absorb some neutrons and slow the reaction, the reaction is explosive. The first atomic bomb worked by this principle (nuclear fission). A more accurate name for both this and the hydrogen bomb ( nuclear fusion) would be "nuclear bomb" or "nuclear weapon", because only the nuclei participate.
Applications
Before radiation was discovered, uranium was primarily used in small amounts for yellow glass and pottery dyes (such as uranium glass and in Fiestaware.) There was also some use in photographic chemicals (esp. uranium nitrate.) It was used in filaments for lamps and in the leather and wood industries for stains and dyes. Uranium salts are mordants of silk or wool. Uranium was also used to improve the appearance of dentures. After the discovery of uranium radiation, additional scientific and practical values of uranium were pursued.
After the discovery in 1939 that it could undergo nuclear fission, uranium gained importance with the development of practical uses of nuclear energy. The first atomic bomb used in warfare, " Little Boy", was a uranium bomb. This bomb contained enough of the uranium-235 isotope to start a runaway chain reaction which in a fraction of a second caused a large number of the uranium atoms to undergo fission, thereby releasing a fireball of energy.
The main use of uranium in the civilian sector is to fuel commercial nuclear power plants. Generally this is in the form of enriched uranium, which has been processed to have higher-than-natural levels of 235U and can be used for a variety of purposes relating to nuclear fission. Commercial nuclear power plants use fuel typically enriched to 2–3% 235U, though some reactor designs (such as the Candu reactors) can use natural uranium (unenriched, less than 1% 235U) fuel. Fuel used for United States Navy submarine reactors is typically highly enriched in 235U (the exact values are classified information). When uranium is enriched over 85% it is known as "weapons grade". In a breeder reactor, 238U can also be converted into plutonium.
Currently the major application of uranium in the U.S. military sector is in high-density penetrators. This ammunition consists of depleted uranium alloyed with 1–2% other elements. The applications of these armor-piercing rounds range from the 20 mm Phalanx gun of the U.S. Navy for piercing attacking missiles, through the 30 mm gun in A-10 aircraft, to 105mm and larger tank barrels. At high impact speed, the density, hardness, and flammability of the projectile enable destruction of heavily armored targets. Tank armour and the removable armour on combat vehicles are also hardened with depleted uranium (DU) plates. The use of DU became a contentious political-environmental issue after US, UK and other countries' use of DU munitions in wars in the Persian Gulf and the Balkans raised questions of uranium compounds left in the soil.
Other uses include:
- The long half-life of the isotope 238U (4.51 × 109 years) make it well-suited for use in estimating the age of the earliest igneous rocks and for other types of radiometric dating (including uranium-thorium dating and uranium-lead dating).
- Uranyl acetate, UO2(CH3COO)2 is used in analytical chemistry. It forms an insoluble salt with sodium.
- Uranium metal is used for X-ray targets in the making of high-energy X-rays.
- Its high atomic mass makes 238U suitable for radiation shielding.
- It is alloyed with iron to make “ferrouranium” that imparts special properties to steels by increasing elastic limit and tensile strength and as a cathode in photoelectric tubes responsive to ultraviolet radiation.
- Distinctive 234U/238U activity ratios (ARs) are a useful environmental tracer of sources of ground water to discharge springs.
- It is a more powerful deoxidiser than vanadium and will denitrogenise steel.
- It is used in high-speed steels as an alloying agent to improve strength and toughness.
- Depleted uranium (uranium with the percentage of 235U lowered to 0.2%) has found use as counterweights for aircraft control surfaces, as ballast for missile re-entry vehicles and as a shielding material. Due to its high density, this material has also found use in inertial guidance devices and in gyroscopic compasses.
History
The use of uranium, in its natural oxide form, dates back to at least CE 79, when it was used to add a yellow colour to ceramic glazes (yellow glass with 1% uranium oxide was found near Naples, Italy). When this was rediscovered, in the earlier part of the 19th century, the world’s only known source of uranium 'earths' were the old Habsburg silver mines in Joachimsthal, Bohemia, and the local glassmaking industry kept a tight lid on the secret ingredient and its supply as long as it could.
The discovery of the element is credited to the German chemist Martin Heinrich Klaproth, who in 1789 found uranium in a mineral called pitchblende. It was named after Uranus the planet, which had been discovered eight years earlier by William Herschel. It was first isolated as a metal in 1841 by Eugene-Melchior Peligot. In 1850 the first commercial use of Uranium in glass was developed by Lloyd & Summerfield of Birmingham, England. Uranium was found to be radioactive by French physicist Henri Becquerel in 1896, who first discovered the process of radioactivity with uranium minerals.
During the Manhattan Project, the wartime Allied program to develop the first atomic bombs during World War II, the United States government bought up many reserves of uranium around the world, although the process of enriching it to applicable levels required gargantuan facilities (see Oak Ridge National Laboratory). Eventually enough uranium, mainly from the Democratic Republic of the Congo (Belgian Congo), was enriched for one atomic bomb nicknamed " Little Boy", which was dropped on Hiroshima, Japan on August 6th, 1945. The other nuclear weapons developed during the war used plutonium as their fissionable material, which itself requires uranium to produce. Initially it was believed that uranium was relatively rare, and that nuclear proliferation could be avoided by simply buying up all known uranium stocks, though within a decade large deposits of it were discovered in many places around the world.
During the Manhattan Project, the names tuballoy and oralloy were used to refer to natural uranium and enriched uranium respectively, originally for purposes of secrecy. These names are still used occasionally to refer to natural or enriched uranium. Less commonly, 25 was used to refer to Uranium-235 by scientists at the Project. The names Q-metal, depletalloy, and D-38, once applied to depleted uranium, have fallen into disuse.
70% of the world's known uranium is located in Australia. The Australian government is currently advocating an expansion of uranium mining, although issues with state governments and indigenous interests complicate the issue.
Bacterial biochemistry
It has been shown in some recent work at Manchester that bacteria can reduce and fix uranium in soils.
Occurrence
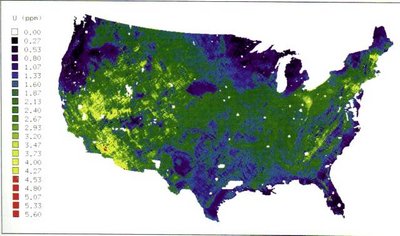
Uranium is a naturally occurring element found in low levels within all rock, soil, and water. This is the highest-numbered element to be found naturally in significant quantities on earth.
It is considered to be more plentiful than antimony, beryllium, cadmium, gold, mercury, silver, or tungsten and is about as abundant as arsenic or molybdenum. It is found in many minerals including uraninite (also called pitchblende, most common uranium ore), autunite, uranophane, torbernite, and coffinite. Significant concentrations of uranium occur in some substances such as phosphate rock deposits, and minerals such as lignite, and monazite sands in uranium-rich ores (it is recovered commercially from these sources).
The decay of uranium, thorium and potassium-40 in the Earth's mantle is thought to be the main source of heat that keeps the outer core liquid and drives mantle convection, which in turn drives plate tectonics.
Uranium ore is rock containing uranium mineralisation in concentrations that can be mined economically, typically 1 to 4 pounds of uranium oxide per ton or 0.05 to 0.20 percent uranium oxide.
Production and distribution
Commercial-grade uranium can be produced through the reduction of uranium halides with alkali or alkaline earth metals. Uranium metal can also be made through electrolysis of KUF5 or UF4, dissolved in a molten CaCl2 and NaCl. Very pure uranium can be produced through the thermal decomposition of uranium halides on a hot filament.
Owners and operators of U.S. civilian nuclear power reactors purchased from U.S. and foreign suppliers a total of 21,300 tons of uranium deliveries during 2001. The average price paid was $26.39 per kilogram of uranium, a decrease of 16 percent compared with the 1998 price. In 2001, the U.S. produced 1,018 tons of uranium from seven mining operations, all of which are west of the Mississippi River.
The ultimate supply of uranium is very large. It is estimated that for a ten times increase in price, the supply of uranium that can be economically mined is increased 300 times.
Uranium exploration and mining
Uranium is distributed worldwide. The world's largest single uranium deposit is located at the Olympic Dam Mine in South Australia.
Australia has the world's largest uranium reserves — 40 percent of the planet's known supply. Almost all the uranium is exported, but under strict International Atomic Energy Agency safeguards to satisfy the Australian people and government that none of the uranium is used in nuclear weapons. Australian uranium is used strictly for electricity production.
In spite of Australia's huge reserves, Canada remains the largest exporter of uranium ore, with mines located in the Athabasca Basin in northern Saskatchewan. Cameco, the world’s largest, low-cost uranium producer accounting for 18% of the world’s uranium production, operates three mines in the area.
There are also significant ore finds in Sweden but it is currently not legal to exploit them.
U.S mining has been in a slump due to the presence of former weapons material available for reprocessing into fuel; the stockpiles of former Soviet uranium and the CES countries' need for dollars; and the start of production at huge high-grade uranium mines in Canada are depressing the market price.
Compounds
Uranium tetrafluoride (UF4) is known as "green salt" and is an intermediate product in the production of uranium hexafluoride. It has the appearance of an emerald-green solid.
Uranium hexafluoride (UF6) is a colorless crystalline solid which forms a vapor at temperatures above 56.4 °C. UF6 is the compound of uranium used for the two most common enrichment processes, gaseous diffusion enrichment, and gas centrifuge enrichment. It is simply called "hex" in the industry. It is corrosive to many metals and reacts violently to water and oils.
Yellowcake is uranium concentrate. It takes its name from the colour and texture of the concentrates produced by early mining operations, despite the fact that modern mills using higher calcining temperatures produce "yellowcake" that is dull yellow to almost black. Initially, the compounds formed in yellowcakes were not identified; in 1970, the U.S. Bureau of Mines still referred to yellowcakes as the final precipitate formed in the milling process and considered it to be ammonium diuranate or sodium diuranate. The compositions were variable and depended upon precipitating conditions. Among the compounds identified in yellowcakes include: uranyl hydroxide, uranyl sulfate, sodium para-uranate, and uranyl peroxide, along with various uranium oxides. Modern yellowcake typically contains 70 to 90 percent uranium oxide (U3O8) by weight. (Other uranium oxides, such as UO2 and UO3, exist; the most stable oxide, U3O8, is actually considered to be a 1:2 molar mixture of these.)
Uranium dioxide a dark brown, crystalline powder, once used in the late 1800s to mid-1900s in ceramic glazes is now used mainly as nuclear fuel, specifically in the form of fuel rods.
Uranyl nitrate (UO2(NO3)2) is an extraordinarily toxic, soluble uranium salt. It appears as a yellow crystalline solid.
Uranium rhodium germanium (URhGe) is the first discovered alloy that becomes superconducting in the presence of an extremely strong electromagnetic field.
Uranium carbonate (UO2(CO3)) is found in both the mineral and organic fractions of coal and its fly ash and is the main component of uranium in mine tailing seepage water.
Uranium trihydride (UH3) appears as a black powder, is highly reactive, and pyrophoric.
Isotopes
Naturally occurring uranium is composed of three major isotopes, 238U, 235U, and 234U, with 238U being the most abundant (99.3% natural abundance). All three isotopes are radioactive, creating radioisotopes, with the most abundant and stable being 238U with a half-life of 4.5 × 109 years, 235U with a half-life of 7 × 108 years, and 234U with a half-life of 2.5 × 105 years. 238U is an α emitter, decaying through the uranium natural decay series into 206Pb.
Uranium isotopes can be separated to increase the concentration of one isotope relative to another. This process is called "enrichment" (see enriched uranium). To be considered "enriched" the 235U fraction has to be increased to significantly greater than 0.711% (by weight) (typically to levels from 3% to 7%). 235U is typically the main fissile material for nuclear power reactors. Either 235U or 239Pu are used for making nuclear weapons. The process produces huge quantities of uranium that is depleted of 235U and with a correspondingly increased fraction of 238U, called depleted uranium or "DU". To be considered "depleted", the 235U isotope concentration has to have been decreased to significantly less than 0.711% (by weight). Typically the amount of 235U left in depleted uranium is 0.2% to 0.3%. This represents anywhere from 28% to 42% of the original fraction of 235U.
Another way to look at this is as follows: Pressurised Heavy Water Reactors (PHWR) use natural uranium (0.71% fissile material). From Pressurised water reactors (PWRs) of typical design (most USA reactors are PWR) we note the fuel goes in with about 4% 235U and 96% 238U and comes out with about 1% 235U, 1% 239Pu and 95% 238U. If the 239Pu were removed (fuel reprocessing is not allowed in the USA) and this were added to the depleted uranium then we would have 1.2% fissile material in the reprocessed depleted uranium and at the same time have 1% fissile material in the left over spent fuel. Both of these would be considered "enriched" fuels for a PHWR style reactor.
233U, an artificial isotope, is used as a reactor fuel in India. It has also been tested in nuclear weapons, but the results were unpromising.
Hazards
All isotopes and compounds of uranium are toxic, teratogenic, and radioactive. It has been shown that some compounds of uranium could cause renal damage, but no conclusive evidence has yet been produced.
No deaths are causally associated with prolonged occupational exposure to inhaled uranium compounds . Although accidental inhalation exposure to a high concentration of uranium hexafluoride has resulted in human fatalities, those deaths were not associated with uranium . On the basis of the available data, exposure to environmental uranium or to uranium at levels found at hazardous waste sites will not be lethal to humans.
Radiological effects are generally local because this is the nature of alpha radiation, the primary form from U-238 decay. Uranium compounds in general are poorly absorbed by the lining in the lungs and may remain a radiological hazard indefinitely. Uranyl (UO2+) ions, such as from uranium trioxide or uranyl nitrate and other hexavalent uranium compounds have been shown to cause birth defects and immune system damage in laboratory animals.
Finely-divided uranium metal presents a fire hazard because uranium is pyrophoric, so small grains will ignite spontaneously in air at room temperature.
A person can be exposed to uranium (or its radioactive daughters) by inhaling dust in air or from smoking tobacco products which have been grown using certain phosphate fertilizers, or ingesting water and food. The general population is exposed to uranium primarily through food and water; the average daily intake of uranium from food ranges from 0.07 to 1.1 micrograms per day. The amount of uranium in air is usually very small; however, people who live near government facilities that made or tested nuclear weapons, or facilities that mine or process uranium ore or enrich uranium for reactor fuel, may have increased exposure to uranium. Houses or structures which are over uranium deposits (either natural or man-made slag deposits) may have an increased incidence of exposure to radon gas, a radioactive carcinogen.
Uranium can enter the body when it is inhaled or swallowed, or under rare circumstances it may enter through cuts in the skin. Uranium does not absorb through the skin, and alpha particles released by uranium cannot penetrate the skin, so uranium that is outside the body is much less harmful than it would be if it were inhaled or swallowed. When uranium enters the body it can lead to kidney damage. Uranium itself is not a chemical carcinogen.
Uranium mining carries the danger of airborne radioactive dust and the release of radioactive radon gas and its daughter products (an added danger to the already dangerous activity of all hard rock mining). As a result, without proper ventilation, uranium miners have a dramatically increased risk of later development of lung cancer and other pulmonary diseases. There is also the possible danger of groundwater contamination with the toxic chemicals used in the separation of the uranium ore.