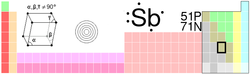
Antimony
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
- Not to be confused with antinomy, a type of paradox.
|
|||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | antimony, Sb, 51 | ||||||||||||||||||||||||
| Chemical series | metalloids | ||||||||||||||||||||||||
| Group, Period, Block | 15, 5, p | ||||||||||||||||||||||||
| Appearance | silvery lustrous grey |
||||||||||||||||||||||||
| Atomic mass | 121.760 (1) g/mol | ||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p3 | ||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 5 | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||
| Density (near r.t.) | 6.697 g·cm−3 | ||||||||||||||||||||||||
| Liquid density at m.p. | 6.53 g·cm−3 | ||||||||||||||||||||||||
| Melting point | 903.78 K (630.63 ° C, 1167.13 ° F) |
||||||||||||||||||||||||
| Boiling point | 1860 K (1587 ° C, 2889 ° F) |
||||||||||||||||||||||||
| Heat of fusion | 19.79 kJ·mol−1 | ||||||||||||||||||||||||
| Heat of vaporization | 193.43 kJ·mol−1 | ||||||||||||||||||||||||
| Heat capacity | (25 °C) 25.23 J·mol−1·K−1 | ||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Crystal structure | rhombohedral | ||||||||||||||||||||||||
| Oxidation states | −3, 3, 5 | ||||||||||||||||||||||||
| Electronegativity | 2.05 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 834 kJ·mol−1 | ||||||||||||||||||||||||
| 2nd: 1594.9 kJ·mol−1 | |||||||||||||||||||||||||
| 3rd: 2440 kJ·mol−1 | |||||||||||||||||||||||||
| Atomic radius | 145 pm | ||||||||||||||||||||||||
| Atomic radius (calc.) | 133 pm | ||||||||||||||||||||||||
| Covalent radius | 138 pm | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Magnetic ordering | no data | ||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 417 nΩ·m | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) 24.4 W·m−1·K−1 | ||||||||||||||||||||||||
| Thermal expansion | (25 °C) 11.0 µm·m−1·K−1 | ||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 3420 m/s | ||||||||||||||||||||||||
| Young's modulus | 55 GPa | ||||||||||||||||||||||||
| Shear modulus | 20 GPa | ||||||||||||||||||||||||
| Bulk modulus | 42 GPa | ||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||
| Brinell hardness | 294 MPa | ||||||||||||||||||||||||
| CAS registry number | 7440-36-0 | ||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| References | |||||||||||||||||||||||||
Antimony ( IPA: /anˈtɪməni/) is a chemical element in the periodic table that has the symbol Sb ( Latin: stibium, meaning "mark") and atomic number 51. A metalloid, antimony has four allotropic forms. The stable form of antimony is a blue-white metal. Yellow and black antimony are unstable non-metals. Antimony is used in flame-proofing, paints, ceramics, enamels, a wide variety of alloys, electronics, and rubber.
Notable characteristics
Antimony in its elemental form is a silvery white, brittle, fusible, crystalline solid that exhibits poor electrical and heat conductivity properties and vaporizes at low temperatures. A metalloid, antimony resembles a metal in its appearance and physical properties, but does not chemically react as a metal. It is also attacked by oxidizing acids and halogens. Antimony and some of its alloys are unusual in that they expand on cooling.
Estimates of the abundance of antimony in the Earth's crust range from 0.2 to 0.5 ppm. Antimony is geochemically categorized as a chalcophile, occurring with sulfur and the heavy metals lead, copper, and silver.
Applications
Antimony is increasingly being used in the semiconductor industry in the production of diodes, infrared detectors, and Hall-effect devices. As an alloy, this semi-metal greatly increases lead's hardness and mechanical strength. The most important use of antimony metal is as a hardener in lead for storage batteries. Uses include:
- Batteries
- antifriction alloys
- type metal
- small arms and tracer bullets
- cable sheathing
- matches
- medicines
- plumbing
- soldering - some "lead-free" solders contain 5% Sb
- main and big-end bearings in internal combustion engines (as alloy)
- used in the past to treat Schistosomiasis; today Praziquantel is universally used
- used in linotype printing machines
Antimony compounds in the form of oxides, sulfides, sodium antimonate, and antimony trichloride are used in the making of flame-proofing compounds, ceramic enamels, glass, paints, and pottery. Antimony trioxide is the most important of the antimony compounds and is primarily used in flame-retardant formulations. These flame-retardant applications include such markets as children's clothing, toys, aircraft and automobile seat covers. Also, antimony sulfide is one of the ingredients of a modern match.
The natural sulfide of antimony, stibnite, was known and used in Biblical times as medicine and as a cosmetic. Stibnite is still used in some developing countries as medicine. Antimony has been used for the treatment of schistosomiasis. Antimony attaches itself to sulfur atoms in certain enzymes which are used by both the parasite and human host. Small doses can kill the parasite without causing damage to the patient. Antimony and its compounds are used in several veterinary preparations like Anthiomaline or Lithium antimony thiomalate, which is used as a skin conditioner in ruminants. Antimony has a nourishing or conditioning effect on keratinized tissues, at least in animals. Tartar emetic is another antimony preparation which is used as an anti-schistosomal drug. Treatments chiefly involving antimony have been called antimonials.
A coin made of antimony was issued in the Keichow Province of China in 1931. The coins were not popular, being too soft and they wore quickly when in circulation. After the first issue no others were produced.
History
Antimony was recognized in antiquity ( 3000 BC or earlier) in various compounds, and it was prized for its fine casting qualities.
The word Antimony is a Latin corruption of Arabic انتيمون ([al-]ithmīd), which is derived from Latin Stibium, which came from Greek στιβι [stibi] = a cosmetic powder (Sb2S3 was used for cosmetic purposes). The relationship between antimony's modern name and its symbol is complex; the Coptic name for the cosmetic powder antimony sulfide was borrowed by the Greeks, which was in turn borrowed by Latin, resulting in stibium. The chemical pioneer Jöns Jakob Berzelius used an abbreviation of this name for antimony in his writings, and his usage became the standard symbol. A black antimony-based powder soluble in water known as stibium was the ancient version of mascara during Roman times. It was used to darken the brows and lashes, or to draw a line around the perimeter of the eye.
According to the history of metallurgy the first description of the procedure to isolate antimony is in the Italian book " De la pirotechnia" of 1540 of Vannoccio Biringuccio. This book precedes the more famous Latin book " De re metallica" of 1556 of Agricola, although the latter has been often incorrectly considered the discoverer of metallic antimony.
According to the traditional history of western alchemy metallic antimony was described (previous to Biringuccio) by the Prior Basilius Valentinus in the Latin manuscript "Currus Triumphalis Antimonii" of about 1450, published, in the English translation "The triumphal chariot of antimony", only in 1604 by Johann Thölde (1565-1614). The marvellous finding of all of the Valentinus' manuscripts, as in the alchemical tales, is fully described by Jean-Jacques Manget in his "Bibliotheca chemica curiosa" (1702): these manuscripts remained enclosed for more than a century in a pillar of St. Peter's Abbey, at Erfurt, until the pillar was shattered by a thunderbolt. Many authors consider Basilius Valentinus a mythological personage: the most authoritative of them is Leibniz (1646-1716), who declared after a careful search that the Prior Valentinus never existed in the Abbey of Erfurt, but was only a pseudonym, probably of Thölde himself, used to merge poorly-translated materials of various origins.
According to the traditional history of Middle Eastern alchemy, pure antimony was well known to Geber, sometimes called "the Father of Chemistry", in the 8th century. Here there is still an open controversy: Marcellin Berthelot, who translated a number of Geber's books, stated that antimony is never mentioned in them, but other authors claim that Berthelot translated only some of the less important books, while the more interesting ones (some of which might describe antimony) are not yet translated, and their content is completely unknown.
Precautions
Antimony and many of its compounds are poisonous. Clinically, antimony poisoning is very similar to arsenic poisoning. In small doses, antimony causes headache, dizziness, and depression. Such small doses have in the past been reported in some acidic fruit drinks. The acidic nature of the drink is sufficient to dissolve small amounts of antimony oxide contained in the packaging of the drink; modern manufacturing methods prevent this occurrence. Larger doses cause violent and frequent vomiting, and will lead to death in a few days.
A study found that antimony is leached from PET bottles, but at levels below drinking water guidelines. The guidelines are:
- WHO, 20 µg l–1
- US EPA, Health Canada and the Ontario Ministry of Environment, 6 µg l–1
- German Federal Ministry of Environment, 5 µg l–1
- Japan, 2 µg l–1
Compounds
Antimony pentafluoride SbF5, antimony trioxide Sb2O3, stibine (antimony trihydride SbH3), indium antimonide (InSb)