Mercury (element)
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
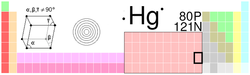
| Name, Symbol, Number | mercury, Hg, 80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 12, 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 200.59 (2) g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | (liquid) 13.534 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 234.32 K (-38.83 ° C, -37.89 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 629.88 K (356.73 ° C, 674.11 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 1750 K, 172.00 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.29 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 59.11 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 27.983 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | rhombohedral | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 1 (mildly basic oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.00 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 1007.1 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1810 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3300 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 150 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 171 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 149 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 155 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (25 °C) 961 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 8.30 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 60.4 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | (liquid, 20 °C) 1451.4 m/s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7439-97-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mercury ( IPA: /ˈmɜːkjəˌɹi/), also called quicksilver, is a chemical element in the periodic table that has the symbol Hg ( Latinized Greek: hydrargyrum, meaning watery or liquid silver) and atomic number 80. A heavy, silvery transition metal, mercury is one of five elements that are liquid at or near standard room temperature (the others are the metals caesium, francium, and gallium, and the nonmetal bromine). Mercury is used in thermometers, barometers and other scientific apparatus, although the use of mercury in thermometers has been largely phased out in clinical and scientific environments (in favour of alcohol-filled, digital or thermistor-based replacements) due to concerns about the element's toxicity. Mercury is still used in dental amalgam. Mercury is mostly obtained by reduction from the mineral cinnabar.
Applications
Mercury is used primarily for the manufacture of industrial chemicals or for electrical and electronic applications. It is used in some thermometers, especially ones which are used to measure high temperatures (In the United States, non-prescription sale of mercury fever thermometers is banned by a number of different states and localities). Other uses:
- Mercury sphygmomanometers.
- Mercury barometers, diffusion pumps, coulometers, and many other laboratory instruments. As an opaque liquid with a very high density, it is ideal for this role.
- The triple point of mercury, -38.8344 °C, is a fixed point used as a temperature standard for the International Temperature Scale ( ITS-90).
- In some gaseous electron tubes, mercury arc rectifier
- Gaseous mercury is used in mercury-vapor lamps and some " neon sign" type advertising signs and fluorescent lamps.
- Liquid mercury was sometimes used as a coolant for nuclear reactors. However, sodium is proposed for reactors cooled with liquid metal, because the high density of mercury requires much energy for circulating the coolant.
- Mercury was once used in the amalgamation process of refining gold and silver ores. This polluting practice is still used by the garimpeiros (gold miners) of the Amazon basin in Brazil.
- Mercury is still used in some cultures for folk medicine and ceremonial purposes which may involve ingestion, injection, or the sprinkling of elemental mercury around the home. It must be emphasized that the former two procedures, especially, are extremely hazardous.
- Alexander Calder built a mercury fountain for the Spanish Pavilion at the 1937 World's Fair in Paris. The fountain is now on display at the Fundació Miró in Barcelona.
- Used in electrochemistry as part of a secondary reference electrode called the calomel electrode as an alternative to the Standard Hydrogen Electrode. This is used to work out the electrode potential of half cells.
Miscellaneous uses: mercury switches, electrodes in some types of electrolysis, batteries ( mercury cells, including for sodium hydroxide and chlorine production, and alkaline batteries), catalysts, insecticides, dental amalgams/preparations and liquid mirror telescopes.
- Thiomersal, (called Thimerosal in the United States), an organic compound used as a preservative in vaccines, though this use is disappearing.
Historical uses: preserving wood, developing daguerreotypes, silvering mirrors, anti-fouling paints (discontinued in 1990), herbicides (discontinued in 1995), cleaning, and in-road leveling devices in cars. Mercury compounds have been used in antiseptics, laxatives, antidepressants, and antisyphilitics. It was also allegedly used by allied spies to sabotage German planes. A mercury paste was applied to bare aluminium, causing the metal to rapidly corrode. This would cause mysterious structural failures.
In Islamic Spain it was used for filling decorative pools and for fountains.
In some applications, mercury can be replaced with less toxic but considerably more expensive galinstan alloy.
A new type of atomic clock, using mercury instead of caesium, has been demonstrated. Accuracy is expected to be within one second in 100 million years.
History
Mercury was known to the ancient Chinese and Hindus and was found in Egyptian tombs that date from 1500 BC. In China, India and Tibet, mercury use was thought to prolong life, heal fractures, and maintain generally good health. China's first emperor, Qin Shi Huang Di — said to have been buried in a tomb that contained rivers of flowing mercury, representative of the rivers of China — was driven insane and killed by mercury pills intended to give him eternal life. The ancient Greeks used mercury in ointments and the Romans used it in cosmetics. By 500 BCE mercury was used to make amalgams with other metals. The Indian word for alchemy is Rasavātam which means ‘the way of mercury’. Alchemists often thought of mercury as the first matter from which all metals were formed. Different metals could be produced by varying the quality and quantity of sulfur contained within the mercury. An ability to transform mercury into any metal resulted from the essentially mercurial quality of all metals. The purest of these was gold, and mercury was required for the transmutation of base (or impure) metals into gold. This was a primary goal of alchemy, either for material or spiritual gain.
Hg is the modern chemical symbol for mercury. It comes from hydrargyrum, a Latinized form of the Greek word `Υδραργυρος (hydrargyros), which is a compound word meaning 'water' and 'silver' — since it is liquid, like water, and yet has a silvery metallic sheen. The element was named after the Roman god Mercury, known for speed and mobility. It is associated with the planet Mercury. The astrological symbol for the planet is also one of the alchemical symbols for the metal (above left). Mercury is the only metal for which the alchemical planetary name became the common name.
Hatting
From the mid-18th to the mid-19th centuries, a process called "carroting" was used in the making of felt hats. Animal skins were rinsed in an orange solution of the mercury compound mercuric nitrate, Hg(NO3)2·2H2O. This process separated the fur from the pelt and matted it together. This solution and the vapors it produced were highly toxic. Its use resulted in widespread cases of mercury poisoning among hatters. Symptoms included tremors, emotional lability, insomnia, dementia and hallucinations. The United States Public Health Service banned the use of mercury in the felt industry in December 1941. The psychological symptoms associated with mercury poisoning may have inspired the phrase "mad as a hatter"; see the hatter article on the origin of the phrase.
Dentistry
Elemental mercury is the main ingredient in dental amalgams. Controversy over the health effects from the use of mercury amalgams began shortly after its introduction into the western world, nearly 200 years ago. In 1843, The American Society of Dental Surgeons, concerned about mercury poisoning, required its members to sign a pledge that they would not use amalgam. In 1859, The American Dental Association was formed by dentists who believed amalgam was "safe and effective." The ADA "continues to believe that amalgam is a valuable, viable and safe choice for dental patients," as written in their statement on dental amalgam. In 1993, the United States Public Health Service reported that "amalgam fillings release small amounts of mercury vapor," but in such a small amount that it "has not been shown to cause any … adverse health effects." This position is not shared by all governments and there is an ongoing dental amalgam controversy. A recent review by an FDA appointed advisory panel, the panelists, in a 13-7 vote, rejected the current FDA report on amalgam safety stating the report's conclusions weren't reasonable, given the quantity and quality of information currently available. Panelists said remaining uncertainties about the risk of so-called silver fillings demanded further research, in particular, on the effects of mercury-laden fillings on children and the fetuses of pregnant women with fillings and the release of mercury vapor on insertion and removal of mercury fillings.
Medicine
Mercury and its compounds have been used in medicine for centuries, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely known and understood.
Mercury(I) chloride (also known as calomel or mercurous chloride) has traditionally been used as a diuretic, topical disinfectant, and laxative. Mercury(II) chloride (also known as mercuric chloride or corrosive sublimate) was once used to treat syphilis (along with other mercury compounds), although it is so toxic that sometimes the symptoms of its toxicity were confused with those of the syphilis it was believed to treat (Pimple 2004); it was also used as a disinfectant. Blue mass, a pill or syrup in which mercury is the main ingredient, was prescribed throughout the 1800s for numerous conditions including constipation, depression, child-bearing and toothaches. In the early 20th century, mercury was administered to children yearly as a laxative and dewormer, and it was used in teething powders for infants. The mercury containing organohalide Mercurochrome is still widely used but has been banned in some countries such as the U.S.
Some vaccines have contained the preservative Thimerosal (partly ethyl mercury) since the 1930s FDA report. It has been widely speculated that this mercury-based preservative can trigger autism in children who are already genetically predisposed to it; however, medical evidence in recent studies has shown no evidence supporting any such link.
Mercury in the form of one of its common ores, cinnabar, remains an important component of Chinese, Tibetan, and Ayurvedic medicine. As problems may arise when these medicines are exported to countries that prohibit the use of mercury in medicines, in recent times, less toxic substitutes have been devised.
Today, the use of mercury in medicine has greatly declined in all respects, especially in developed countries. Thermometers and sphygmomanometers containing mercury were invented in the early 18th and late 19th centuries, respectively. In the early 21st century, their use is declining and has been banned in some countries, states and medical institutions. In 2002, the U.S. Senate passed legislation to phase out the sale of non-prescription mercury thermometers. In 2003, Washington and Maine became the first states to ban mercury blood pressure devices. Mercury compounds are found in some over-the-counter drugs, including topical antiseptics, stimulant laxatives, diaper-rash ointment, eye drops, and nasal sprays. The FDA (FDA) has “inadequate data to establish general recognition of the safety and effectiveness,” of the mercury ingredients in these products. Mercury is still used in some diuretics, although substitutes now exist for most therapeutic uses.
In the European Union, RoHS legislation being introduced will ban mercury from certain products, and limit the amount of mercury in other products to less than 1000 ppm (except for certain exemptions).
Mineral occurrence
Mercury is an extremely rare element in the earth's crust, having an average crustal abundance by mass of only 0.08 parts per million. However, because it does not blend geochemically with those elements that comprise the majority of the crustal mass, mercury ores can be extraordinarily concentrated considering the element's abundance in ordinary rock. The richest mercury ores contain up to 2.5% mercury by mass, and even the leanest concentrated deposits are at least 0.1% mercury (12,000 times average crustal abundance). This makes mercury ore the most easily depleted of all metal ores. Depletion of mercury ores has been a major concern since the 1960s and it is now almost certain that the last mineable deposits were discovered in Algeria in the mid-1970s. Since the early 1970s, total world production of mercury has fallen from 9,000 tons to 1,600 tons due to depletion of reserve.
It is found either as a native metal (rare) or in cinnabar, corderoite, livingstonite, and other minerals with cinnabar (HgS) being the most common ore. Mercury ores usually occur in very young orogenic belts where rock of high density are forced to the crust of the Earth, often in hot springs or other volcanic regions. Most present-day production occurs in Spain, Kyrgyzstan, China and Tajikistan. Over 100,000 tons of mercury were mined from the region of Huancavelica, Peru, over the course of three centuries following the discovery of deposits there in 1563; mercury from Huancavelica was crucial in the production of silver in colonial Spanish America. Many former ores in Italy, Slovenia, the United States and Mexico which once produced a large proportion of the world's supply have now been completely mined out. The metal is extracted by heating cinnabar in a current of air and condensing the vapor. The equation for this extraction is
- HgS + O2 → Hg + SO2
Compounds
The most important salts are:
- Mercury(I) chloride (AKA calomel) is sometimes still used in medicine and acousto-optical filters
- Mercury(II) chloride (which is very corrosive, sublimates and is a violent poison)
- Mercury fulminate, (a detonator widely used in explosives),
- Mercury(II) oxide, the main oxide of mercury
- Mercury(II) sulfide (AKA cinnabar mercuric ore still used in oriental medicine, or vermilion which is a high-grade paint pigment),
- Mercury(II) selenide a semiconductor,
- Mercury(II) telluride a semiconductor, and
- Mercury cadmium telluride and mercury zinc telluride, infrared detector materials.
Laboratory tests have found that an electrical discharge causes the noble gases to combine with mercury vapor. These compounds are held together with van der Waals forces and result in HgNe, HgAr, HgKr, and HgXe. Organic mercury compounds are also important. Methylmercury is a dangerous compound that is widely found as a pollutant in water bodies and streams.
Isotopes
There are seven stable isotopes of mercury with Hg-202 being the most abundant (29.86%). The longest-lived radioisotopes are 194Hg with a half-life of 444 years, and 203Hg with a half-life of 46.612 days. Most of the remaining radioisotopes have half-lifes that are less than a day.
Reactivity
Dissolves to form amalgams with gold, zinc and many metals, but with some exceptions such as iron, therefore iron flasks have been traditionally used to trade mercury. Reacts with oxygen in air when heated to form mercury oxide, which then can be decomposed by further heating to higher temperatures. Being below hydrogen in the reactivity series of metals, it does not react with most acids, such as dilute sulfuric acid, though oxidizing acids, such as concentrated sulfuric acid and nitric acid or aqua regia dissolve it to give sulfate and nitrate and chloride. Similar to silver, mercury reacts with atmospheric hydrogen sulfide. Mercury even reacts with solid sulfur flakes, which is used in mercury spill kits to absorb mercury vapors (spill kits also use activated charcoal and powdered zinc).
Occurrence in the environment
Abundance
- Crustal ~7×10-2 mg/kg
- Oceans ~3×10-5 mg/l
Preindustrial deposition rates of mercury from the atmosphere may be in the range of 4 ng/L in the western USA. Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.
Mercury enters the environment as a pollutant from various industries:
- coal-fired power plants are the largest source (40% of USA emissions in 1999).
- industrial processes
- medical applications, including vaccinations
- dentistry
- cosmetic industries
- laboratory work involving mercury or sulfur compounds
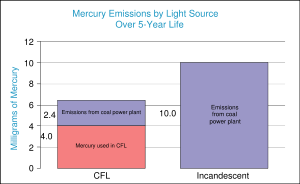
Mercury also enters into the environment through the disposal (e.g., landfilling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats. Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, most thermometers now use pigmented alcohol instead of mercury. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are being replaced by electronic thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
One of the worst industrial disasters in history was caused by the dumping of mercury compounds into Minamata Bay, Japan. The Chisso Corporation, a fertilizer and later petrochemical company, was found responsible for polluting the bay from 1932–1968. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.
Precautions and regulation
Mercury should be handled with care. Containers of mercury should be securely sealed to avoid spills and evaporation. Heating of mercury, or compounds of mercury that may decompose when heated, should always be carried out with adequate ventilation in order to avoid human exposure to mercury vapor. Mercury should never be used as decoration and or displayed in open containers. Most of its compounds are highly toxic, especially its inorganic compounds.
Occupational exposure
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization, OSHA, and NIOSH all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the Environmental Protection Agency.
Mercury in fish
Fish and shellfish have a natural tendency to concentrate mercury in their bodies, often in the form of methylmercury, a highly toxic organic compound of mercury. Species of fish that are high on the food chain, such as shark, swordfish, king mackerel, albacore tuna, and tilefish contain higher concentrations of mercury than others. This is because mercury is stored in the muscle tissues of fish, and when a predatory fish eats another fish, it assumes the entire body burden of mercury in the consumed fish. Since fish are less efficient at depurating than accumulating methylmercury, fish-tissue concentrations increase over time. Thus species that are high on the food chain amass body burdens of mercury that can be ten times higher, or more, than the species they consume. This process is called biomagnification. The complexities associated with mercury fate and transport are relatively succinctly described by USEPA in their 1997 Mercury Study Report to Congress. Because methylmercury and high levels of elemental mercury can be particularly toxic to unborn or young children, organizations such as the U.S. EPA and FDA recommend that women who are pregnant or plan to become pregnant within the next one or two years, as well as young children avoid eating more than 6 ounces (one average meal) of fish per week. In the United States the FDA has an action level for methyl mercury in commercial marine and freshwater fish that is 1.0 parts per million (ppm), and in Canada the limit for the total of mercury content is 0.5 (ppm).
A common warning
Species with characteristically low levels of mercury include shrimp, tilapia, salmon, pollock, and catfish (FDA March 2004). The FDA characterizes shrimp, catfish, pollock, salmon, and canned light tuna as low-mercury seafood, although recent tests have indicated that up to 6 percent of canned light tuna may contain high levels.
The danger of avoiding fish
The effects of consuming fish high in mercury is in dispute with the University of Rochester's study of people in the Republic of the Seychelles. While there is no doubt high level exposure to methyl mercury is definitely toxic, low level exposure isn't. A recent Harvard Medical School study of mothers and their infants suggests that the nutritional benefits of fish outweigh the effects of mercury. In the HMS study, each additional weekly serving of fish consumed by the mother during pregnancy was associated with an increase in infant cognition.
What one Professor Emeritus of Engineering (University of Denver) says about mercury in fish:
Recently (December 2003), news reports stated that tuna fish contained "large amounts of mercury" in an alarming tone. What they really should have said is that tuna fish contains only extremely small, scarcely detectable, amounts of methyl mercury. The extrapolated threat appears to be to the nerves of developing fetuses. I wonder how many cases have been observed of fetal mercury damage by normal tuna fish. I suspect that the number is zero. The authorities probably have no idea of the magnitude of the hazard, only that a hazard could be possible. Perhaps we have homeopaths at work here! Generally, they deal in extrapolations of inexact data to minute concentrations, and probably do not know the rate at which organisms take up and excrete such tiny quantities. The problem is that methyl mercury could be a threat, but I have no confidence that the authorities know enough about it to protect the public, only to enjoy the creation of panic. Tuna fish, indeed, also contains many nutrients valuable to fetuses.
Release of mercury into the environment

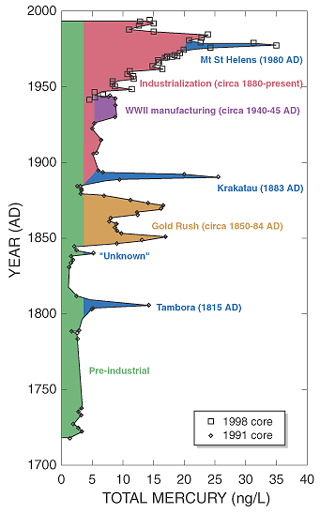
Historically, one of the largest releases was from the Colex plant, a Lithium isotope separation plant at Oak Ridge. The plant operated in the 1950's and 1960's. Records are incomplete and unclear, but govt commissions have estimated 2 million pounds of mercury are unaccounted for.
The primary sources of mercury to the environment are fossil fuel burning (primarily coal) and solid waste incineration (Nriagu & Pacyna, 1988). Power plants in the U.S., according to U.S. Environmental Protection Agency, are one of the main emitters of mercury — 48 tonnes a year
The United States Clean Air Act, passed in 1990, put mercury on a list of toxic pollutants which need to be controlled to the greatest possible extent. Thus, certain industries that emit mercury into the environment must install maximum achievable control technologies (MACT). However, a March 2005 EPA rule took power plants off the list of sources which must reduce mercury to the maximum extent. Instead, a cap and trade rule was issued, with most of the reductions in mercury pollution from power plants beginning in the year 2018. States were also given until November, 2006 to impose stricter controls, and several states are doing so. The rule was being subjected to legal challenges from several states in 2005.
Mercury and aluminium
Mercury readily combines with aluminium to form an amalgam when the two pure metals come into contact. However, when the amalgam is exposed to air, the aluminium oxidizes, leaving behind mercury. The oxide flakes away, exposing more mercury amalgam, which repeats the process. This process continues until the supply of amalgam is exhausted, and since it releases mercury, a small amount of mercury can “eat through” a large amount of aluminium over time, by progressively forming amalgam and relinquishing the aluminium as oxide.
Aluminium in air is ordinarily protected by a molecule-thin layer of its own oxide (which is not porous to oxygen). Mercury coming into contact with this oxide does no harm. However, if any elemental aluminium is exposed (even by a recent scratch), the mercury may combine with it, starting the process described above, and potentially damaging a large part of the aluminium before it finally ends (Ornitz 1998).
For this reason, restrictions are placed on the use and handling of mercury in proximity with aluminium. In particular, mercury is not allowed aboard aircraft under most circumstances because of the risk of it forming amalgam with exposed aluminium parts in the aircraft.
Reclamation of mercury mines
Due to minimal surface disruption, mercury mines lend themselves to constructive re-use. For example, in 1976 the Santa Clara County, California purchased the historic Almaden Quicksilver Mine and proceeded to create a county park on the site, after conducting extensive safety and environmental analysis of the property.