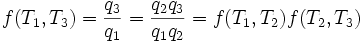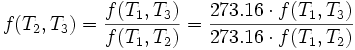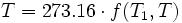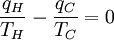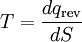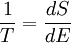Thermodynamic temperature
2007 Schools Wikipedia Selection. Related subjects: General Physics
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an “absolute” scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the lowest possible temperature where nothing could be colder.
Overview
Temperature arises from the random submicroscopic vibrations of the particle constituents of matter. These motions comprise the kinetic energy in a substance. More specifically, the thermodynamic temperature of any bulk quantity of matter is the measure of the average kinetic energy of a certain kind of vibrational motion of its constituent particles called translational motions. Translational motions are ordinary, whole-body movements in 3D space whereby particles move about and exchange energy in collisions. Fig. 1 at right shows translational motion in gases; Fig. 4 below shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature at which the particle constituents of matter have minimal motion, retaining only quantum mechanical motion.
Throughout the scientific world where measurements are made in SI units, thermodynamic temperature is measured in kelvins (symbol: K). Many engineering fields in the U.S. measure thermodynamic temperature using the Rankine scale.
By international agreement, the unit “kelvin” and its scale are defined by two points: absolute zero, and the triple point of specially prepared (VSMOW) water. Absolute zero—the coldest possible temperature—is defined as being precisely 0 K and −273.15 °C. The triple point of water is defined as being precisely 273.16 K and 0.01 °C. This definition does three things: 1) it fixes the magnitude of the kelvin unit as being precisely 1 part in 273.16 parts the difference between absolute zero and the triple point of water; 2) it establishes that one kelvin has precisely the same magnitude as a one-degree increment on the Celsius scale; and 3) it establishes the difference between the two scales’ null points as being precisely 273.15 kelvins (0 K = −273.15 °C and 273.16 K = 0.01 °C). Conversion from kelvins to degrees Rankine (°R) is accomplished as follows: TK × 1.8 = T°R.
Table of thermodynamic temperatures
The full range of the thermodynamic temperature scale and some notable points along it are shown in the table below.
| kelvin | Celsius | Peak emittance wavelength of black-body photons |
|
| Absolute zero (precisely by definition) |
0 K | −273.15 °C | ∞ |
| Coldest measured temperature |
450 pK | –273.149 999 999 55 °C | 6,400 kilometers |
| One millikelvin (precisely by definition) |
0.001 K | −273.149 °C | 2.897 77 meters (Radio, FM band) |
| Water’s triple point (precisely by definition) |
273.16 K | 0.01 °C | 10,608.3 nm (Long wavelength I.R.) |
| Water’s boiling point A | 373.1339 K | 99.9839 °C | 7766.03 nm (Mid wavelength I.R.) |
| Incandescent lampB | 2500 K | ≈2200 °C | 1160 nm (Near infrared)C |
| Sun’s visible surfaceD | 5778 K | 5505 °C | 501.5 nm ( Green light) |
| Lightning bolt’s channel E |
28,000 K | 28,000 °C | 100 nm (Far Ultraviolet light) |
| Sun’s core E | 16 MK | 16 million °C | 0.18 nm ( X-rays) |
| Thermonuclear weapon (peak temperature)E |
350 MK | 350 million °C | 8.3 × 10−3 nm ( Gamma rays) |
| Sandia National Labs’ Z machine E |
2 GK | 2 billion °C | 1.4 × 10−3 nm (Gamma rays)F |
| Core of a high–mass star on its last day E |
3 GK | 3 billion °C | 1 × 10−3 nm (Gamma rays) |
| Merging binary neutron star system E |
350 GK | 350 billion °C | 8 × 10−6 nm (Gamma rays) |
| Relativistic Heavy Ion Collider E |
1 TK | 1 trillion °C | 3 × 10−6 nm (Gamma rays) |
| CERN’s proton vs. nucleus collisions E |
10 TK | 10 trillion °C | 3 × 10−7 nm (Gamma rays) |
| Universe 5.391 × 10−44 s after the Big Bang E |
1.417 × 1032 K | 1.417 × 1032 °C | 1.616 × 10−26 nm (Planck frequency) |
A For Vienna Standard Mean Ocean Water at one standard atmosphere (101.325 kPa) when calibrated strictly per the two-point definition of thermodynamic temperature.
B The 2500 K value is approximate. The 273.15 K difference between K and °C is rounded to 300 K to avoid false precision in the Celsius value.
C For a true blackbody (which tungsten filaments are not). Tungsten filaments’ emissivity is greater at shorter wavelengths which makes them appear whiter.
D Effective photosphere temperature. The 273.15 K difference between K and °C is rounded to 273 K to avoid false precision in the Celsius value.
E The 273.15 K difference between K and °C is ignored to avoid false precision in the Celsius value.
F For a true blackbody (which the plasma was not). The Z machine’s dominant emission originated from 40 MK electrons (soft x–ray emissions) within the plasma.
The relationship of temperature, motions, conduction, and heat energy
The nature of kinetic energy, translational motion, and temperature
At its simplest, “temperature” arises from the kinetic energy of the vibrational motions of matter’s particle constituents ( molecules, atoms, and subatomic particles). The full variety of these kinetic motions contribute to the total heat energy in a substance. The relationship of kinetic energy, mass, and velocity is given by the formula Ek = 1/2m • v 2. Accordingly, those particles with one unit of mass moving at one unit of velocity have the same kinetic energy—and the same temperature—as those with twice the mass but only 70.7% of the velocity.
The thermodynamic temperature of any bulk quantity of a substance (a statistically significant quantity of particles) is directly proportional to the average—or “mean”—kinetic energy of a specific kind of particle motion known as translational motion. These simple movements in the three x, y, and z–axis dimensions of space means the particles move in the three spatial degrees of freedom. This particular form of kinetic energy is sometimes referred to as kinetic temperature. Translational motion is but one form of heat energy and is what gives gases not only their temperature, but also their pressure and the vast majority of their volume. This relationship between the temperature, pressure, and volume of gases is established by the ideal gas law’s formula pV = nRT.
The extent to which the kinetic energy of translational motion of an individual atom or molecule (particle) in a gas contributes to the pressure and volume of that gas is a proportional function of thermodynamic temperature as established by the Boltzmann constant (symbol: kB). The Boltzmann constant also relates the thermodynamic temperature of a gas to the mean kinetic energy of an individual particle’s translational motion as follows:
- Emean = 3/2kBT
- where…
- Emean = joules (symbol: J)
- kB = 1.380 6505(24) × 10−23 J/K
- T = thermodynamic temperature in kelvins
- Emean = joules (symbol: J)
- where…
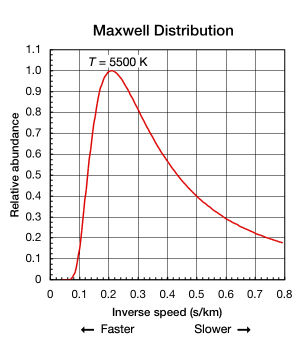
While the Boltzmann constant is useful for finding the mean kinetic energy of a particle, it’s important to note that even when a substance is isolated and in thermodynamic equilibrium (all parts are at a uniform temperature and no heat is going into or out of it), the translational motions of individual atoms and molecules occurs across a wide range of speeds (see animation in Fig. 1 above). At any one instant, the proportion of particles moving at a given speed within this range is determined by probability as described by the Maxwell–Boltzmann distribution. The graph shown here in Fig. 2 shows the speed distribution of 5500 K helium atoms. They have a most probable speed of 4.780 km/s (0.2092 s/km). However, a certain proportion of atoms at any given instant are moving faster while others are moving relatively slowly; some are momentarily at a virtual standstill (off the x–axis to the right). This graph uses inverse speed for its x–axis so the shape of the curve can easily be compared to the curves in Fig. 5 below. In both graphs, zero on the x–axis represents infinite temperature. Additionally, the x and y–axis on both graphs are scaled proportionally.
The high speeds of translational motion
Although very specialized laboratory equipment is required to directly detect translational motions, the resultant collisions by atoms or molecules with small particles suspended in a fluid produces Brownian motion that can be seen with an ordinary microscope. The translational motions of elementary particles are very fast and temperatures close to absolute zero are required to directly observe them. For instance, when scientists at the NIST achieved a record-setting cold temperature of 700 nK (billionths of a kelvin) in 1994, they used optical lattice laser equipment to adiabatically cool cesium atoms. They then turned off the entrapment lasers and directly measured atom velocities of 7 mm per second to in order to calculate their temperature. Formulas for calculating the velocity and speed of translational motion are given in the following footnote.
The internal motions of molecules and specific heat
There are other forms of heat energy besides the kinetic energy of translational motion. As can be seen in the animation at right, molecules are complex objects; they are a population of atoms and thermal agitation can strain its internal chemical bonds in three different ways: via rotation, bond length, and bond angle movements. These are all types of internal degrees of freedom. This makes molecules distinct from monatomic substances (consisting of individual atoms) like the noble gases helium and argon, which have only the three translational degrees of freedom. Kinetic energy is stored in molecules’ internal degrees of freedom, which gives them an internal temperature. Even though these motions are called “internal,” the external portions of molecules still move—rather like the jiggling of a stationary water balloon. This permits the two-way exchange of kinetic energy between internal motions and translational motions with each molecular collision. Accordingly, as heat is removed from molecules, both their kinetic temperature (the kinetic energy of translational motion) and their internal temperature simultaneously diminish in equal proportions. This phenomenon is described by the equipartition theorem, which states that for any bulk quantity of a molecular-based substance in equilibrium, the kinetic energy of particle motion is evenly distributed among all the active degrees of freedom available to the particles.
The kinetic energy stored internally in molecules does not contribute directly to the temperature of a substance (nor to the pressure or volume of gases). This is because any kinetic energy that is, at a given instant, bound in internal motions is not at that same instant contributing to the molecules’ translational motions. Since the internal temperature of the molecules in any bulk quantity of a substance in equilibrium is, on average, equal to their kinetic temperature, the distinction is usually of interest only in the detailed study of non-equilibrium phenomena such as the sublimation of solids and the diffusion of hot gases in a partial vacuum.
Different molecules absorb different amounts of heat energy for each incremental increase in temperature. Water for instance, can absorb a large amount of heat energy per mole (a specific number of particles) with only a modest temperature change. This property is known as a substance’s specific heat capacity. High specific heat capacity arises, in part, because certain substance’s molecules possess more internal degrees of freedom than others. For instance, room-temperature nitrogen, which is a diatomic molecule, has five active degrees of freedom: the three comprising translational motion plus two rotational degrees of freedom internally. Not surprisingly, in accordance to the equipartition theorem, nitrogen has five-thirds the molar heat capacity as do the monatomic gases. Larger, more complex molecules can have dozens of internal degrees of freedom.
The diffusion of heat energy: Entropy, phonons, and mobile conduction electrons
Heat conduction is the diffusion of heat energy from hot parts of a system to cold. A “system” can be either a single bulk entity or a plurality of discrete bulk entities. The term “bulk” in this context means a statistically significant quantity of particles (which can be a microscopic amount). Anytime heat energy diffuses within an isolated system, temperature differences within the system decrease (entropy increases).
One particular heat conduction mechanism occurs when translational motion—the particle motion underlying temperature—transfers momentum from particle to particle in collisions. In gases, these translational motions are of the nature shown above in Fig. 1. As can be seen in that animation, not only does momentum (heat) diffuse throughout the volume of the gas through serial collisions, but entire molecules or atoms can advance forward into new territory, bringing their kinetic energy with them. Consequently, heat diffuses through gases rather easily; especially for light atoms or molecules. Convection speeds this process even more.
Translational motion in solids however, takes the form of phonons (see Fig. 4 at right). Phonons are constrained, quantized wave packets traveling at the speed of sound for a given substance. The manner in which phonons interact within a solid determines a variety of its properties, including its thermal conductivity. In electrically insulating solids, phonon-based heat conduction is usually inefficient and such solids are considered to be thermal insulators (such as glass, plastic, rubber, ceramic, and rock). This is because in solids, atoms and molecules are locked into place relative to their neighbors and are not free to roam.
Metals however, are not restricted to only phonon-based heat conduction. Heat energy conducts through metals extraordinarily quickly because instead of direct molecule-to-molecule collisions, the vast majority of heat energy is mediated via very light, mobile conduction electrons. This is why there is a near-perfect correlation between metals’ thermal conductivity and their electrical conductivity. Conduction electrons imbue metals with their extraordinary conductivity because they are delocalized, i.e. not tied to a specific atom, and behave rather like a sort of “quantum gas” due to the effects of zero-point energy (for more on ZPE, see Note 1 below). Furthermore, electrons are relatively light with a rest mass only 1/1836th that of a proton. This is about the same ratio as a .22 Short bullet (29 grains or 1.88 g) compared to the rifle that shoots it. As Sir Isaac Newton once wrote with his third law of motion:
- “Law #3: All forces occur in pairs, and these two forces
- are equal in magnitude and opposite in direction.”
However, a bullet accelerates faster than a rifle given an equal force. Since kinetic energy increases as the square of velocity, nearly all the kinetic energy goes into the bullet, not the rifle, even though both experience the same force from the expanding propellant gases. In the same manner—because they are much less massive—heat energy is readily borne by mobile conduction electrons. Too, because they’re delocalized and very fast, kinetic heat energy conducts extremely quickly through metals with abundant conduction electrons.
The diffusion of heat energy: Black-body radiation
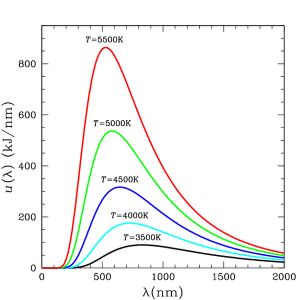
Thermal radiation is a byproduct of the collisions arising from atoms’ various vibrational and rotational motions. These collisions cause atoms to emit thermal photons (known as black-body radiation). Photons are emitted anytime an electric charge is accelerated (as happens when two atoms’ electron clouds collide). Even individual molecules with internal temperatures greater than absolute zero also emit black-body radiation from their atoms. In any bulk quantity of a substance at equilibrium, black-body photons are emitted across a range of wavelengths in a spectrum that has a bell curve-like shape called a Planck curve (see graph in Fig. 5 at right). The top of a Planck curve— the peak emittance wavelength—is located in particular part of the electromagnetic spectrum depending on the temperature of the black body. Substances at extreme cryogenic temperatures emit at long radio wavelengths whereas extremely hot temperatures produce short gamma rays (see Table of thermodynamic temperatures, above).
Black-body radiation diffuses heat energy throughout a substance as the photons are absorbed by neighboring atoms, transferring momentum in the process. Black-body photons also easily escape from a substance and can be absorbed by the ambient environment; kinetic energy is lost in the process.
As established by the Stefan–Boltzmann law, the intensity of black-body radiation increases as the fourth power of absolute temperature. Thus, a black body at 824 K (just short of glowing dull red) emits 60 times the radiant power as it does at 296 K (room temperature). This is why one can so easily feel the radiant heat from hot objects at a distance. At higher temperatures, such as those found in an incandescent lamp, black-body radiation can be the principal mechanism by which heat energy escapes a system.
The heat of phase changes
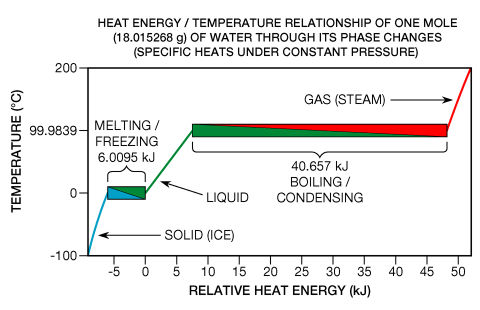
The kinetic energy of particle motion is just one contributor to the total heat energy in a substance. The other is the potential energy of molecular bonds that can yet form in a substance as it cools (such as during condensing and freezing). This concept may be more easily grasped by visualizing it in the reverse direction: as the heat energy required to break molecular bonds (such as during evaporation and melting). These processes are known as phase transitions. The heat energy required for a phase transition is called latent heat. Anyone who has compared the 100 °C air from a hair dryer to 100 °C steam knows that the steam can quickly cause severe burns whereas the air can not. The burn occurs because a large amount of heat energy is liberated as steam condenses into liquid water on the skin. Even though heat energy is liberated or absorbed during phase transitions, pure chemical elements, compounds, and eutectic alloys exhibit no temperature change whatsoever while they undergo them (see Fig. 7, below right).
This phenomenon can be readily understood by examining one particular type of phase transition: the melting of a solid. When a solid melts, crystal lattice chemical bonds break apart; the substance has gone from what is known as a more ordered state to a less ordered state (see Topological order). In Fig. 7, the melting of ice is shown within the lower left box heading from blue to green. At one specific thermodynamic point, the melting point (which is 0 °C across a wide pressure range in the case of water), all the atoms or molecules are—on average—at the maximum energy threshold the lattice bonds can withstand without breaking and jumping to a higher quantum energy state.
Quantum transitions are a complete jump from one energy level to another; no intermediate values are possible. Consequently, when a substance is at its melting point, every joule of heat energy that is added to it only breaks bonds of a specific quantity of its atoms or molecules, releasing them from the crystal lattice and converting them into a liquid of precisely the same temperature; no kinetic energy is added to translational motion (which is what gives substances their temperature). The effect is rather like popcorn: at a certain temperature, additional heat energy can’t make the kernels any hotter until the transition (popping) is complete. If the process is reversed (as in the freezing of a liquid), heat energy must be removed from a substance.
As stated above, the heat energy required for a phase transition is called latent heat. In the specific cases of melting and freezing, it’s called enthalpy of fusion or heat of fusion. If the molecular bonds in a crystal lattice are strong, the heat of fusion can be relatively great, typically in the range of 6 to 30 kJ per mole for water and most of the metallic elements. If the substance is one of the monatomic gases, (which have little tendency to form molecular bonds) the heat of fusion is more modest, ranging from 0.021 to 2.3 kJ per mole. Relatively speaking, phase transitions can be truly energetic events. To completely melt ice at 0 °C into water at 0 °C, one must add roughly 80 times the heat energy as is required to increase the temperature of the same mass of liquid water by one degree Celsius. The metals’ ratios are even greater, typically in the range of 400 to 1200 times. And the phase transition of boiling is much more energetic than freezing. For instance, the energy required to completely boil or vaporize water (what is known as enthalpy of vaporization) is roughly 540 times that required for a one-degree increase. Water’s sizable enthalpy of vaporization is why one’s skin can be burned so quickly as steam condenses on it (heading from red to green in Fig. 7 above). In the opposite direction, this is why one’s skin feels cool as liquid water on it evaporates (a process that occurs at a sub-ambient wet-bulb temperature that is dependent on relative humidity).
Internal energy
The total kinetic energy of all particle motion — including that of conduction electrons — plus the potential energy of phase changes, plus zero-point energy comprise the internal energy of a substance, which is its total heat energy. The term internal energy mustn’t be confused with internal degrees of freedom. Whereas the internal degrees of freedom of molecules refers to one particular place where kinetic energy is bound, the internal energy of a substance is composed of heat energy in all its various forms.
Heat energy at absolute zero
As a substance cools, many forms of heat energy and their related effects simultaneously decrease in magnitude: the latent heat of available phase transitions are liberated as a substance changes from a less ordered state to a more ordered state; the translational motions of atoms and molecules diminish (their kinetic temperature decreases); the internal motions of molecules diminish (their internal temperature decreases); conduction electrons (if the substance is an electrical conductor) travel somewhat slower; and black-body radiation’s peak emittance wavelength increases (the photons’ energy decreases). When the particles of a substance are as close as possible to complete rest and retain only quantum mechanical motion, the substance is at the temperature of absolute zero (T=0).
Note that whereas absolute zero is the point of zero temperature, absolute zero is not necessarily the point at which a substance contains zero heat energy; one must be very precise with what one means by “heat energy.” Often, all the phase changes that can occur in a substance, will have occurred by the time it reaches absolute zero. However, this is not always the case. For instance, T=0 helium remains liquid at room pressure and must be under a pressure of at least 25 bar to crystallize. This is because helium’s heat of fusion — think of it as the energy required to melt helium ice — is so low (only 21 J mol−1) that the motion-inducing effect of zero-point energy is sufficient to prevent it from freezing at lower pressures. If helium is cooled while under at least 25 bar of pressure, this latent heat energy is liberated as the helium freezes while approaching absolute zero.
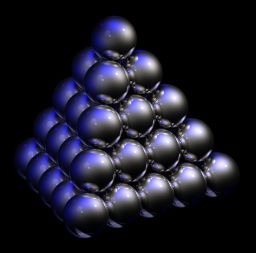
Further complicating matters is that many solids change their crystal structure to more compact arrangements at extremely high pressures (up to millions of bars). These are known as solid-solid phase transitions wherein heat is liberated as a crystal lattice changes to a more thermodynamically favorable, compact one. These complexities make for rather cumbersome blanket statements regarding the internal energy in T=0 substances. Regardless of pressure though, what can be said is that at absolute zero, all solids with a lowest-energy crystal lattice such those with a closest-packed arrangement (see Fig. 8, above left) contain minimal internal energy, retaining only that due to the ever-present background of zero-point energy. One can also say that for all substances at any fixed pressure, absolute zero is the point of minimal enthalpy (a measure of heat content that takes pressure into consideration). Lastly, it is always true to say that all T=0 substances have zero kinetic heat energy.
The origin of heat energy
Earth’s proximity to the Sun is why most everything near Earth’s surface is warm with a temperature substantially above absolute zero. The Sun constantly replenishes heat energy the Earth loses into space. Because matter is everywhere in the natural world, and because of the wide variety of heat diffusion mechanisms (one of which is black-body radiation which occurs at the speed of light), objects on Earth rarely vary too far from the global mean surface and air temperature of 287 to 288 K (14 to 15 °C). The more an object’s or system’s temperature varies from this average, the more rapidly it tends to come back into equilibrium with the ambient environment.
History of thermodynamic temperature
- 1702–1703: Guillaume Amontons (1663 – 1705) published two papers that credit him with being the first researcher to deduce the existence of a fundamental (thermodynamic) temperature scale featuring an absolute zero. He made the discovery while endeavoring to improve upon the air thermometers in use at the time. His J-tube thermometers comprised a mercury column that was supported by a fixed mass of air entrapped within the sensing portion of the thermometer. In thermodynamic terms, his thermometers relied upon the volume / temperature relationship of gas under constant pressure. His measurements of the boiling point of water and the melting point of ice showed that regardless of the mass of air trapped inside his thermometers or the weight of mercury the air was supporting, the reduction in air volume at the ice point was always the same ratio. This observation lead him to posit that a sufficient reduction in temperature would reduce the air volume to zero. In fact, his calculations projected that absolute zero was equivalent to −240 degrees on today’s Celsius scale—only 33.15 degrees short of the true value of −273.15 °C.
- 1742: Anders Celsius (1701 – 1744) created a “backwards” version of the modern Celsius temperature scale whereby zero represented the boiling point of water and 100 represented the melting point of ice. In his paper Observations of two persistent degrees on a thermometer, he recounted his experiments showing that ice’s melting point was effectively unaffected by pressure. He also determined with remarkable precision how water’s boiling point varied as a function of atmospheric pressure. He proposed that zero on his temperature scale (water’s boiling point) would be calibrated at the mean barometric pressure at mean sea level.
- 1744: Coincident with the death of Anders Celsius, the famous botanist Carolus Linnaeus (1707 – 1778) effectively reversed Celsius’s scale upon receipt of his first thermometer featuring a scale where zero represented the melting point of ice and 100 represented water’s boiling point. His custom-made “linnaeus-thermometer,” for use in his greenhouses, was made by Daniel Ekström, Sweden’s leading maker of scientific instruments at the time. For the next 204 years, the scientific and thermometry communities world-wide referred to this scale as the “centigrade scale.” Temperatures on the centigrade scale were often reported simply as “degrees” or, when greater specificity was desired, “degrees centigrade.” The symbol for temperature values on this scale was °C (in several formats over the years). Because the term “centigrade” was also the French-language name for a unit of angular measurement (one-hundredth of a right angle) and had a similar connotation in other languages, the term “centesimal degree” was used when very precise, unambiguous language was required by international standards bodies such as the Bureau international des poids et mesures (BIPM). The 9th CGPM ( Conférence générale des poids et mesures) and the CIPM ( Comité international des poids et mesures) formally adopted “degree Celsius” (symbol: °C) in 1948.
- 1777: In his book Pyrometrie (Berlin: Haude & Spener, 1779) completed four months before his death, Johann Heinrich Lambert (1728 – 1777)—sometimes incorrectly referred to as Joseph Lambert—proposed an absolute temperature scale based on the pressure / temperature relationship of a fixed volume of gas. This is distinct from the volume / temperature relationship of gas under constant pressure that Guillaume Amontons discovered 75 years earlier. Lambert stated that absolute zero was the point where a simple straight-line extrapolation reached zero gas pressure and was equal to −270 °C.
- Circa 1787: Notwithstanding the work of Guillaume Amontons 85 years earlier, Jacques Alexandre César Charles (1746 – 1823) is often credited with “discovering”, but not publishing, that the volume of a gas under constant pressure is proportional to its absolute temperature. The formula he created was V1/T1 = V2/T2.
- 1802: Joseph Louis Gay-Lussac (1778 – 1850) published work (acknowledging the unpublished lab notes of Jacques Charles fifteen years earlier) describing how the volume of gas under constant pressure changes linearly with its absolute (thermodynamic) temperature. This behaviour is called Charles’s Law and is one of the gas laws. His are the first known formulas to used the number “273” for the expansion coefficient of gas relative to the melting point of ice (indicating that absolute zero was equivalent to −273 °C).
- 1848:William Thomson, (1824 – 1907) also known as Lord Kelvin, wrote in his paper, On an Absolute Thermometric Scale, of the need for a scale whereby “infinite cold” (absolute zero) was the scale’s null point, and which used the degree Celsius for its unit increment. As did Gay-Lussac, Thomson calculated that absolute zero was equivalent to −273 °C on the air thermometers of the time. This absolute scale is known today as the Kelvin thermodynamic temperature scale. It’s noteworthy that Thomson’s value of “−273” was actually derived from 0.00366, which was the accepted expansion coefficient of gas per degree Celsius relative to the ice point. The inverse of −0.00366 expressed to four significant digits is −273.2 °C which is remarkably close to the true value of −273.15 °C.
- 1859: William John Macquorn Rankine (1820 – 1872) proposed a thermodynamic temperature scale similar to William Thomson’s but which used the degree Fahrenheit for its unit increment. This absolute scale is known today as the Rankine thermodynamic temperature scale.
- 1877 - 1884: Ludwig Boltzmann (1844 – 1906) made major contributions to thermodynamics through an understanding of the role that particle kinetics and black-body radiation played. His name is now attached to several of the formulas used today in thermodynamics.
- Circa 1930s: Gas thermometry experiments carefully calibrated to the melting point of ice and boiling point of water showed that absolute zero was equivalent to −273.15 °C.
- 1948: Resolution 3 of the 9th CGPM (Conférence Générale des Poids et Mesures, also known as the General Conference on Weights and Measures) fixed the triple point of water at precisely 0.01 °C. At this time, the triple point still had no formal definition for its equivalent kelvin value, which the resolution declared “will be fixed at a later date.” The implication is that if the value of absolute zero measured in the 1930s was truly −273.15 °C, then the triple point of water (0.01 °C) was equivalent to 273.16 K. Also, both the CIPM (Comité international des poids et mesures, also known as the International Committee for Weights and Measures) and the CGPM formally adopted the name “Celsius” for the “degree Celsius” and the “Celsius temperature scale.”
- 1954: Resolution 3 of the 10th CGPM gave the Kelvin scale its modern definition by choosing the triple point of water as its second defining point and assigned it a temperature of precisely 273.16 kelvin (what was actually written 273.16 “degrees Kelvin” at the time). This, in combination with Resolution 3 of the 9th CGPM, had the effect of defined absolute zero as being precisely zero kelvin and −273.15 °C.
- 1967/1968: Resolution 3 of the 13th CGPM renamed the unit increment of thermodynamic temperature “kelvin”, symbol K, replacing “degree absolute”, symbol °K. Further, feeling it useful to more explicitly define the magnitude of the unit increment, the 13th CGPM also decided in Resolution 4 that “The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.”
- 2005: The CIPM (Comité International des Poids et Mesures, also known as the International Committee for Weights and Measures) affirmed that for the purposes of delineating the temperature of the triple point of water, the definition of the Kelvin thermodynamic temperature scale would refer to water having an isotopic composition defined as being precisely equal to the nominal specification of VSMOW water.
Derivations of thermodynamic temperature
Strictly speaking, the temperature of a system is well-defined only if its particles (atoms, molecules, electrons, photons) are at equilibrium, and so obey a Boltzmann distribution (or its quantum mechanical counterpart). There are many possible scales of temperature, derived from a variety of observations of physical phenomena. The thermodynamic temperature can be shown to have special properties, and in particular can be seen to be uniquely defined ( up to some constant multiplicative factor) by considering the efficiency of idealized heat engines. Thus the ratios of temperatures, T2/T1, are the same in all absolute scales.
Loosely stated, temperature controls the flow of heat between two systems and the Universe, as we would expect any natural system, tends to progress so as to maximize entropy. Thus, we would expect there to be some relationship between temperature and entropy. In order to find this relationship let's first consider the relationship between heat, work and temperature. A heat engine is a device for converting heat into mechanical work and analysis of the Carnot heat engine provides the necessary relationships we seek. The work from a heat engine corresponds to the difference between the heat put into the system at the high temperature, qH and the heat ejected at the low temperature, qC. The efficiency is the work divided by the heat put into the system or:
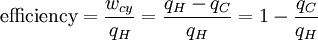 (1)
(1)
where wcy is the work done per cycle. We see that the efficiency depends only on qC/qH. Because qC and qH correspond to heat transfer at the temperatures TC and TH, respectively, qC/qH should be some function of these temperatures:
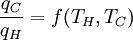 (2)
(2)
Carnot's theorem states that all reversible engines operating between the same heat reservoirs are equally efficient. Thus, a heat engine operating between T1 and T3 must have the same efficiency as one consisting of two cycles, one between T1 and T2, and the second between T2 and T3. This can only be the case if:
Consequently, we have:
where T1 is the temperature of the triple point of water. So we can define the thermodynamic temperature as:
This temperature scale has the property that:
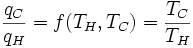 (3)
(3)
Substituting Equation 3 back into Equation 1 gives a relationship for the efficiency in terms of temperature:
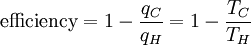 (4)
(4)
Notice that for TC=0 K the efficiency is 100% and that efficiency becomes greater than 100% below 0 K. Since an efficiency greater than 100% violates the first law of thermodynamics, this requires that 0 K must be the minimum possible temperature. This makes intuitive sense; since temperature is the motion of particles, no system can, on average, have less motion than the minimum permitted by quantum physics. In fact, as of June 2006, the coldest man-made temperature was 450 pK. Subtracting the right hand side of Equation 4 from the middle portion and rearranging gives:
where the negative sign indicates heat ejected from the system. This relationship suggests the existence of a state function, S, defined by:
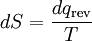 (5)
(5)
where the subscript indicates a reversible process. The change of this state function around any cycle is zero, as is necessary for any state function. This function corresponds to the entropy of the system, which we described previously. We can rearranging Equation 5 to get a new definition for temperature in terms of entropy and heat:
For a system, where entropy S may be a function S(E) of its energy E, the thermodynamic temperature T is given by:
The reciprocal of the thermodynamic temperature is the rate of increase of entropy with energy.