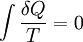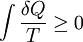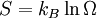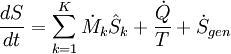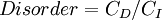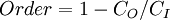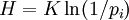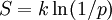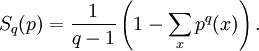Entropy
2007 Schools Wikipedia Selection. Related subjects: General Physics
The concept of entropy in thermodynamics is central to the second law of thermodynamics, which deals with physical processes and whether they occur spontaneously. Spontaneous changes occur with an increase in entropy. In contrast the first law of thermodynamics deals with the concept of energy, which is conserved. Entropy change has often been defined as a change to a more disordered state at a microscopic level. In recent years, entropy has been interpreted in terms of the " dispersal" of energy. Entropy is an extensive state function that accounts for the effects of irreversibility in thermodynamic systems.
Quantitatively, entropy, symbolized by S, is defined by the differential quantity dS = δQ / T, where δQ is the amount of heat absorbed in a reversible process in which the system goes from one state to another, and T is the absolute temperature. Entropy is one of the factors that determines the free energy of the system.
When a system's energy is defined as the sum of its "useful" energy, (e.g. that used to push a piston), and its "useless energy", i.e. that energy which cannot be used for external work, then entropy may be (most concretely) visualized as the "scrap" or "useless" energy whose energetic prevalence over the total energy of a system is directly proportional to the absolute temperature of the considered system, as is the case with the Gibbs free energy or Helmholtz free energy relations.
In terms of statistical mechanics, the entropy describes the number of the possible microscopic configurations of the system. The statistical definition of entropy is generally thought to be the more fundamental definition, from which all other important properties of entropy follow. Although the concept of entropy was originally a thermodynamic construct, it has been adapted in other fields of study, including information theory, psychodynamics, thermoeconomics, and evolution.
History
The short history of entropy begins with the work of mathematician Lazare Carnot who in his 1803 work Fundamental Principles of Equilibrium and Movement postulated that in any machine the accelerations and shocks of the moving parts all represent losses of moment of activity. In other words, in any natural process there exists an inherent tendency towards the dissipation of useful energy. Building on this work, in 1824 Lazare's son Sadi Carnot published Reflections on the Motive Power of Fire in which he set forth the view that in all heat-engines whenever " caloric", or what is now known as heat, falls through a temperature difference, that work or motive power can be produced from the actions of the "fall of caloric" between a hot and cold body. This was an early insight into the second law of thermodynamics.
Carnot based his views of heat partially on the early 18th century "Newtonian hypothesis" that both heat and light were types of indestructible forms of matter, which are attracted and repelled by other matter, and partially on recent 1789 views of Count Rumford who showed that heat could be created by friction as when cannons bored. Accordingly, Carnot reasoned that if the body of the working substance, such as a body of steam, is brought back to its original state (temperature and pressure) at the end of a complete engine cycle, that "no change occurs in the condition of the working body." This latter comment was amended in his foot notes, and it was this comment that led to the development of entropy.
In the 1850s and 60s, German physicist Rudolf Clausius gravely objected to this latter supposition, i.e. that no change occurs in the working body, and gave this "change" a mathematical interpretation by questioning the nature of the inherent loss of usable heat when work is done, e.g., heat produced by friction. This was in contrast to earlier views, based on the theories of Isaac Newton, that heat was an indestructible particle that had mass. Later, scientists such as Ludwig Boltzmann, Willard Gibbs, and James Clerk Maxwell gave entropy a statistical basis. Carathéodory linked entropy with a mathematical definition of irreversibility, in terms of trajectories and integrability.
Definitions and descriptions
In science, the term "entropy" is generally interpreted in three distinct, but semi-related, ways, i.e. from macroscopic viewpoint ( classical thermodynamics), a microscopic viewpoint ( statistical thermodynamics), and an information viewpoint ( information theory). Entropy in information theory is a fundamentally different concept from thermodynamic entropy. However, at a philosophical level, some argue that thermodynamic entropy can be interpreted as an application of the information entropy concept to a very particular set of physical questions.
Macroscopic viewpoint (classical thermodynamics)
| Conjugate variables of thermodynamics |
|
|---|---|
| Pressure | Volume |
| ( Stress) | ( Strain) |
| Temperature | Entropy |
| Chem. potential | Particle no. |
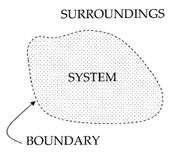
In a thermodynamic system, a "universe" consisting of "surroundings" and "systems" and made up of quantities of matter, its pressure differences, density differences, and temperature differences all tend to equalize over time. In the ice melting example, the difference in temperature between a warm room (the surroundings) and cold glass of ice and water (the system and not part of the room), begins to be equalized as portions of the heat energy from the warm surroundings become spread out to the cooler system of ice and water.
Over time the temperature of the glass and its contents and the temperature of the room become equal. The entropy of the room has decreased and some of its energy has been dispersed to the ice and water. However, as calculated in the example, the entropy of the system of ice and water has increased more than the entropy of the surrounding room has decreased. In an isolated system such as the room and ice water taken together, the dispersal of energy from warmer to cooler always results in a net increase in entropy. Thus, when the 'universe' of the room and ice water system has reached a temperature equilibrium, the entropy change from the initial state is at a maximum. The entropy of the thermodynamic system is a measure of how far the equalization has progressed.
A special case of entropy increase, the entropy of mixing, occurs when two or more different substances are mixed. If the substances are at the same temperature and pressure, there will be no net exchange of heat or work - the entropy increase will be entirely due to the mixing of the different substances.
From a macroscopic perspective, in classical thermodynamics the entropy is interpreted simply as a state function of a thermodynamic system: that is, a property depending only on the current state of the system, independent of how that state came to be achieved. The state function has the important property that, when multiplied by a reference temperature, it can be understood as a measure of the amount of energy in a physical system that cannot be used to do thermodynamic work; i.e., work mediated by thermal energy. More precisely, in any process where the system gives up energy ΔE, and its entropy falls by ΔS, a quantity at least TR ΔS of that energy must be given up to the system's surroundings as unusable heat (TR is the temperature of the system's external surroundings). Otherwise the process will not go forward.
In 1862, Clausius stated what he calls the “theorem respecting the equivalence-values of the transformations” or what is now known as the second law of thermodynamics, as such:
- The algebraic sum of all the transformations occurring in a cyclical process can only be positive, or, as an extreme case, equal to nothing.
Quantitatively, Clausius states the mathematical expression for this theorem is as follows. Let δQ be an element of the heat given up by the body to any reservoir of heat during its own changes, heat which it may absorb from a reservoir being here reckoned as negative, and T the absolute temperature of the body at the moment of giving up this heat, then the equation:
must be true for every reversible cyclical process, and the relation:
must hold good for every cyclical process which is in any way possible. This is the essential formulation of the second law and one of the original forms of the concept of entropy. It can be seen that the dimensions of entropy are energy divided by temperature, which is the same as the dimensions of Boltzmann's constant (k_B) and heat capacity. The SI unit of entropy is " joule per kelvin" (J•K−1). In this manner, the quantity "ΔS" is utilized as a type of internal ordering energy, which accounts for the effects of irreversibility, in the energy balance equation for any given system. In the Gibbs free energy equation, i.e. ΔG = ΔH - TΔS, for example, which is a formula commonly utilized to determine if chemical reactions will occur, the energy related to entropy changes TΔS is subtracted from the "total" system energy ΔH to give the "free" energy ΔG of the system, as during a chemical process or as when a system changes state.
Microscopic viewpoint (statistical mechanics)
From a microscopic perspective, in statistical thermodynamics the entropy is a measure of the number of microscopic configurations that are capable of yielding the observed macroscopic description of the thermodynamic system:
where Ω is the number of microscopic configurations, and kB is Boltzmann's constant. In Boltzmann's 1896 Lectures on Gas Theory, he showed that this expression gives a measure of entropy for systems of atoms and molecules in the gas phase, thus providing a measure for the entropy of classical thermodynamics.
In 1877, thermodynamicist Ludwig Boltzmann visualized a probabilistic way to measure the entropy of an ensemble of ideal gas particles, in which he defined entropy to be proportional to the logarithm of the number of microstates such a gas could occupy. Henceforth, the essential problem in statistical thermodynamics, i.e. according to Erwin Schrödinger, has been to determine the distribution of a given amount of energy E over N identical systems.
Statistical mechanics explains entropy as the amount of uncertainty (or "mixedupness" in the phrase of Gibbs) which remains about a system, after its observable macroscopic properties have been taken into account. For a given set of macroscopic quantities, like temperature and volume, the entropy measures the degree to which the probability of the system is spread out over different possible quantum states. The more states available to the system with higher probability, and thus the greater the entropy. In essence, the most general interpretation of entropy is as a measure of our ignorance about a system. The equilibrium state of a system maximizes the entropy because we have lost all information about the initial conditions except for the conserved quantities; maximizing the entropy maximizes our ignorance about the details of the system.
On the molecular scale, the two definitions match up because adding heat to a system, which increases its classical thermodynamic entropy, also increases the system's thermal fluctuations, so giving an increased lack of information about the exact microscopic state of the system, i.e. an increased statistical mechanical entropy.
Entropy in chemical thermodynamics
Thermodynamic entropy is central in chemical thermodynamics, enabling changes to be quantified and the outcome of reactions predicted. The second law of thermodynamics states that entropy in the combination of a system and its surroundings (or in an isolated system by itself) increases during all spontaneous chemical and physical processes. Spontaneity in chemistry means “by itself, or without any outside influence”, and has nothing to do with speed. The Clausius equation of δqrev/T = ΔS introduces the the measurement of entropy change, ΔS. Entropy change describes the direction and quantitates the magnitude of simple changes such as heat transfer between systems – always from hotter to cooler spontaneously. Thus, when a mole of substance at 0 K is warmed by its surroundings to 298 K, the sum of the incremental values of qrev/T constitute each element's or compound's standard molar entropy, a fundamental physical property and an indicator of the amount of energy stored by a substance at 298 K. Entropy change also measures the mixing of substances as a summation of their relative quantities in the final mixture.
Entropy is equally essential in predicting the extent of complex chemical reactions, i.e. whether a process will go as written or proceed in the opposite direction. For such applications, ΔS must be incorporated in an expression that includes both the system and its surroundings, Δ Suniverse = ΔSsurroundings + Δ S system. This expression becomes, via some steps, the Gibbs free energy equation for reactants and products in the system: Δ G [the Gibbs free energy change of the system] = Δ H [the enthalpy change] – T Δ S [the entropy change].
The second law
An important law of physics, the second law of thermodynamics, states that the total entropy of any isolated thermodynamic system tends to increase over time, approaching a maximum value; and so, by implication, the entropy of the universe (i.e. the system and its surroundings), assumed as an isolated system, tends to increase. Two important consequences are that heat cannot of itself pass from a colder to a hotter body: i.e., it is impossible to transfer heat from a cold to a hot reservoir without at the same time converting a certain amount of work to heat. It is also impossible for any device that can operate on a cycle to receive heat from a single reservoir and produce a net amount of work; it can only get useful work out of the heat if heat is at the same time transferred from a hot to a cold reservoir. This means that there is no possibility of a " perpetual motion" which is isolated. Also, from this it follows that a reduction in the increase of entropy in a specified process, such as a chemical reaction, means that it is energetically more efficient.
In general, according to the second law, the entropy of a system that is not isolated may decrease. An air conditioner, for example, cools the air in a room, thus reducing the entropy of the air. The heat, however, involved in operating the air conditioner always makes a bigger contribution to the entropy of the environment than the decrease of the entropy of the air. Thus the total entropy of the room and the environment increases, in agreement with the second law.
Entropy balance equation for open systems
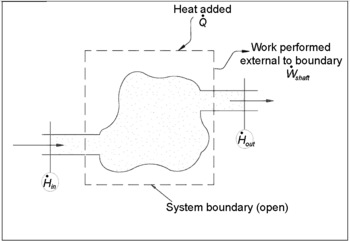
In chemical engineering, the principles of thermodynamics are commonly applied to " open systems", i.e. those in which heat, work, and mass flow across the system boundary. In a system in which there are flows of both heat (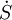 ) and work, i.e.
) and work, i.e. 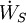 (shaft work) and P(dV/dt) (pressure-volume work), across the system boundaries, the heat flow, but not the work flow, causes a change in the entropy of the system. This rate of entropy change is
(shaft work) and P(dV/dt) (pressure-volume work), across the system boundaries, the heat flow, but not the work flow, causes a change in the entropy of the system. This rate of entropy change is 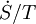 , where T is the absolute thermodynamic temperature of the system at the point of the heat flow. If, in addition, there are mass flows across the system boundaries, the total entropy of the system will also change due to this convected flow.
, where T is the absolute thermodynamic temperature of the system at the point of the heat flow. If, in addition, there are mass flows across the system boundaries, the total entropy of the system will also change due to this convected flow.
To derive a generalized entropy balanced equation, we start with the general balance equation for the change in any extensive quantity Θ in a thermodynamic system, a quantity that may be either conserved, such as energy, or non-conserved, such as entropy. The basic generic balance expression states that dΘ/dt, i.e. the rate of change of Θ in the system, equals the rate at which Θ enters the system at the boundaries, minus the rate at which Θ leaves the system across the system boundaries, plus the rate at which Θ is generated within the system. Using this generic balance equation, with respect to the rate of change with time of the extensive quantity entropy S, the entropy balance equation for an open thermodynamic system is:
where
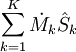 = the net rate of entropy flow due to the flows of mass into and out of the system (where
= the net rate of entropy flow due to the flows of mass into and out of the system (where 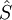 = entropy per unit mass).
= entropy per unit mass).
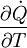 = the rate of entropy flow due to the flow of heat across the system boundary.
= the rate of entropy flow due to the flow of heat across the system boundary.
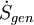 = the rate of internal generation of entropy within the system.
= the rate of internal generation of entropy within the system.
Note, also, that if there are multiple heat flows, the term 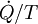 is to be replaced by
is to be replaced by 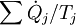 , where
, where 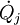 is the heat flow and Tj is the temperature at the jth heat flow port into the system.
is the heat flow and Tj is the temperature at the jth heat flow port into the system.
Standard textbook definitions
- Entropy – energy broken down in irretrievable heat.
- – Boltzmann's constant times the logarithm of a multiplicity; where the multiplicity of a macrostate is the number of microstates that correspond to the macrostate.
- – the number of ways of arranging things in a system (times the Boltzmann's constant).
- – a non-conserved thermodynamic state function, measured in terms of the number of microstates a system can assume, which corresponds to a degradation in usable energy.
- – a direct measure of the randomness of a system.
- – a measure of energy dispersal at a specific temperature.
- – a measure of the partial loss of the ability of a system to perform work due to the effects of irreversibility.
- – an index of the tendency of a system towards spontaneous change.
- – a measure of the unavailability of a system’s energy to do work; also a measure of disorder; the higher the entropy the greater the disorder.
- – a parameter representing the state of disorder of a system at the atomic, ionic, or molecular level.
- – a measure of disorder in the universe or of the availability of the energy in a system to do work.
Approaches to understanding entropy
Order and disorder
Entropy, historically, has often been associated with the amount of order, disorder, and or chaos in a thermodynamic system. The traditional definition of entropy is that it refers to changes in the status quo of the system and is a measure of "molecular disorder" and the amount of wasted energy in a dynamical energy transformation from one state or form to another. In this direction, a number of authors, in recent years, have derived exact entropy formulas to account for and measure disorder and order in atomic and molecular assemblies. One of the simpler entropy order/disorder formulas is that derived in 1984 by thermodynamic physicist Peter Landsberg, which is based on a combination of thermodynamics and information theory arguments. Landsberg argues that when constraints operate on a system, such that it is prevented from entering one or more of its possible or permitted states, as contrasted with its forbidden states, the measure of the total amount of “disorder” in the system is given by the following expression:
Similarly, the total amount of "order" in the system is given by:
In which CD is the "disorder" capacity of the system, which is the entropy of the parts contained in the permitted ensemble, CI is the "information" capacity of the system, an expression similar to Shannon's channel capacity, and CO is the "order" capacity of the system.
Energy dispersal
The concept of entropy can be described qualitatively as a measure of energy dispersal at a specific temperature. Similar terms have been in use from early in the history of classical thermodynamics, and with the development of statistical thermodynamics and quantum theory, entropy changes have been described in terms of the mixing or "spreading" of the total energy of each constituent of a system over its particular quantized energy levels.
Ambiguities in the terms disorder and chaos, which usually have meanings directly opposed to equilibrium, contribute to widespread confusion and hamper comprehension of entropy for most students. As the second law of thermodynamics shows, in an isolated system internal portions at different temperatures will tend to adjust to a single uniform temperature and thus produce equilibrium. A recently developed educational approach avoids ambiguous terms and describes such spreading out of energy as dispersal, which leads to loss of the differentials required for work even though the total energy remains constant in accordance with the first law of thermodynamics. Physical chemist Peter Atkins, for example, who previously wrote of dispersal leading to a disordered state, now writes that "spontaneous changes are always accompanied by a dispersal of energy", and has discarded 'disorder' as a description.
Entropy and Information theory
In information theory, entropy is the measure of the amount of information that is missing before reception and is sometimes referred to as Shannon entropy.. Shannon entropy is a very general concept which finds applications in information theory as well as thermodynamics. It was originally devised by Claude Shannon in 1948 to study the amount of information in a transmitted message. The definition of the information entropy is, however, very general, and is expressed in terms of a discrete set of probabilities pi. In the case of transmitted messages, these probabilities were the probabilities that a particular message was actually transmitted, and the entropy of the message system was a measure of how much information was in the message. For the case of equal probabilities (i.e. each message is equally probable), the Shannon entropy (in bits) is just the number of yes/no questions needed to determine the content of the message.
The question of the link between information entropy and thermodynamic entropy is a hotly debated topic. Many authors argue that there is a link between the two, while others will argue that they have absolutely nothing to do with each other.
The expressions for the two entropies are very similar. The information entropy H for equal probabilities pi is:
where K is a constant which determines the units of entropy. For example, if the units are bits, then K=1/\ln(2). The thermodynamic entropy S , from a statistical mechanical point of view was first expressed by Boltzmann:
where p is the probability of a system being in a particular microstate, given that it is in a particular macrostate, and k is Boltzmann's constant. It can be seen that one may think of the thermodynamic entropy as Boltzmann's constant, divided by ln(2), times the number of yes/no questions that must be asked in order to determine the microstate of the system, given that we know the macrostate. The link between thermodynamic and information entropy was developed in a series of papers by Edwin Jaynes beginning in 1957.
The problem with linking thermodynamic entropy to information entropy is that the entire body of thermodynamics which deals with the physical nature of entropy is missing. The second law of thermodynamics which governs the behaviour of thermodynamic systems in equilibrium, and the first law which expresses heat energy as the product of temperature and entropy are physical concepts rather than informational concepts. If thermodynamic entropy is seen as including all of the physical dynamics of entropy as well as the equilibrium statistical aspects, then information entropy gives only part of the description of thermodynamic entropy. Some authors, like Tom Schneider, argue for dropping the word entropy for the H function of information theory and using Shannon's other term "uncertainty" instead.
Ice melting example
The illustration for this article is a classic example in which entropy increases in a small 'universe', a thermodynamic system consisting of the 'surroundings' (the warm room) and 'system' (glass, ice, cold water). In this universe, some heat energy δQ from the warmer room surroundings (at 298 K or 25 C) will spread out to the cooler system of ice and water at its constant temperature T of 273 K (0 C), the melting temperature of ice. The entropy of the system will change by the amount dS = δQ/T, in this example δQ/273 K. (The heat δQ for this process is the energy required to change water from the solid state to the liquid state, and is called the enthalpy of fusion, i.e. the ΔH for ice fusion.) The entropy of the surroundings will change by an amount dS = -δQ/298 K. So in this example, the entropy of the system increases, whereas the entropy of the surroundings decreases.
It is important to realize that the decrease in the entropy of the surrounding room is less than the increase in the entropy of the ice and water: the room temperature of 298 K is larger than 273 K and therefore the ratio, (entropy change), of δQ/298 K for the surroundings is smaller than the ratio (entropy change), of δQ/273 K for the ice+water system. To find the entropy change of our 'universe', we add up the entropy changes for its constituents: the surrounding room, and the ice+water. The total entropy change is positive; this is always true in spontaneous events in a thermodynamic system and it shows the predictive importance of entropy: the final net entropy after such an event is always greater than was the initial entropy.
As the temperature of the cool water rises to that of the room and the room further cools imperceptibly, the sum of the δQ/T over the continuous range, at many increments, in the initially cool to finally warm water can be found by calculus. The entire miniature "universe", i.e. this thermodynamic system, has increased in entropy. Energy has spontaneously become more dispersed and spread out in that "universe" than when the glass of ice water was introduced and became a "system" within it.
Topics in entropy
Entropy and life
For over a century and a half, beginning with Clausius' 1863 memoir "On the Concentration of Rays of Heat and Light, and on the Limits of its Action", much writing and research has been devoted to the relationship between thermodynamic entropy and the evolution of life. The argument that that life feeds on negative entropy or negentropy as put forth in the 1944 book What is Life? by physicist Erwin Schrödinger served as a further stimulus to this research. Recent writings have utilized the concept of Gibbs free energy to elaborate on this issue. In other cases, some creationists have argued that entropy rules out evolution.
In the popular textbook 1982 textbook Principles of Biochemistry by noted American biochemist Albert Lehninger, for example, it is argued that the order produced within cells as they grow and divide is more than compensated for by the disorder they create in their surroundings in the course of growth and division. In short, according to Lehninger, "living organisms preserve their internal order by taking from their surroundings free energy, in the form of nutrients or sunlight, and returning to their surroundings an equal amount of energy as heat and entropy."
Evolution related definitions:
- Negentropy - a shorthand colloquial phrase for negative entropy.
- Ectropy - a measure of the tendency of a dynamical system to do useful work and grow more organized.
- Syntropy - a tendency towards order and symmetrical combinations and designs of ever more advantageous and orderly patterns.
- Extropy – a metaphorical term defining the extent of a living or organizational system's intelligence, functional order, vitality, energy, life, experience, and capacity and drive for improvement and growth.
- Ecological entropy - a measure of biodiversity in the study of biological ecology.
The arrow of time
Entropy is the only quantity in the physical sciences that "picks" a particular direction for time, sometimes called an arrow of time. As we go "forward" in time, the Second Law of Thermodynamics tells us that the entropy of an isolated system can only increase or remain the same; it cannot decrease. Hence, from one perspective, entropy measurement is thought of as a kind of clock.
Entropy and cosmology
We have previously mentioned that a finite universe may be considered an isolated system. As such, it may be subject to the Second Law of Thermodynamics, so that its total entropy is constantly increasing. It has been speculated that the universe is fated to a heat death in which all the energy ends up as a homogeneous distribution of thermal energy, so that no more work can be extracted from any source.
If the universe can be considered to have generally increasing entropy, then - as Roger Penrose has pointed out - gravity plays an important role in the increase because gravity causes dispersed matter to accumulate into stars, which collapse eventually into black holes. Jacob Bekenstein and Stephen Hawking have shown that black holes have the maximum possible entropy of any object of equal size. This makes them likely end points of all entropy-increasing processes, if they are totally effective matter and energy traps. Hawking has, however, recently changed his stance on this aspect.
The role of entropy in cosmology remains a controversial subject. Recent work has cast extensive doubt on the heat death hypothesis and the applicability of any simple thermodynamic model to the universe in general. Although entropy does increase in the model of an expanding universe, the maximum possible entropy rises much more rapidly and leads to an "entropy gap", thus pushing the system further away from equilibrium with each time increment. Other complicating factors, such as the energy density of the vacuum and macroscopic quantum effects, are difficult to reconcile with thermodynamical models, making any predictions of large-scale thermodynamics extremely difficult.
Other relations
Generalized entropy
Many generalizations of entropy have been studied, two of which, Tsallis and Rényi entropies, are widely used and the focus of active research.
The Rényi entropy is an information measure for fractal systems.
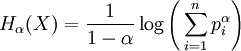 .
.
where α > 0 is the 'order' of the entropy, pi are the probabilities of {x1, x2 ... xn}. For α = 1 we recover the standard entropy form.
The Tsallis entropy is employed in Tsallis statistics to study nonextensive thermodynamics.
where p denotes the probability distribution of interest, and q is a real parameter that measures the non- extensitivity of the system of interest. In the limit as q → 1, we again recover the standard entropy.
Other mathematical definitions
- Kolmogorov-Sinai entropy - a mathematical type of entropy in dynamical systems related to measures of partitions.
- Topological entropy - a way of defining entropy in an iterated function map in ergodic theory.
- Relative entropy - is a natural distance measure from a "true" probability distribution P to an arbitrary probability distribution Q.
Sociological definitions
The concept of entropy has also entered the domain of sociology, generally as a metaphor for chaos, disorder or dissipation of energy, rather than as a direct measure of thermodynamic or information entropy:
- Entropology – the study or discussion of entropy or the name sometimes given to thermodynamics without differential equations.
- Psychological entropy - the distribution of energy in the psyche, which tends to seek equilibrium or balance among all the structures of the psyche.
- Economic entropy – a quantitative measure of the irrevocable dissipation and degradation of natural materials and available energy with respect to economic activity.
- Social entropy – a measure of social system structure, having both theoretical and statistical interpretations, i.e. society (macrosocietal variables) measured in terms of how the individual functions in society (microsocietal variables); also related to social equilibrium.
- Corporate entropy - energy waste as red tape and business team inefficiency, i.e. energy lost to waste.
Quotes & humor
- As stated by John von Neumann in conversation with Claude Shannon in 1949:
| Nobody knows what entropy really is, so in a debate you will always have the advantage. |
- As stated by Frederic Keffer:
| The future belongs to those who can manipulate entropy; those who understand but energy will be only accountants. |