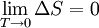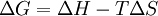Absolute zero
2007 Schools Wikipedia Selection. Related subjects: General Physics
Absolute zero refers to the temperature of a system that is thermically inert. Such a (theoretical) system neither emits nor absorbs heat energy. The Absolute zero temperature is known to be 0 K (-273.15 C). While it is possible to cool any substance to near Absolute Zero, it can never actually be achieved.
Absolute zero is the point at which particles have a minimum energy, determined by quantum mechanical effects, which is called the zero-point energy.
By international agreement, absolute zero is defined as precisely 0 K on the Kelvin scale, which is a thermodynamic (absolute) temperature scale, and -273.15°C on the Celsius scale. Absolute zero is also precisely equivalent to 0 °R on the Rankine scale (also a thermodynamic temperature scale), and –459.67 °F on the Fahrenheit scale.
Whilst scientists cannot fully achieve a state of “zero” heat energy in a substance, they have made great advancements in achieving temperatures ever closer to absolute zero (where matter exhibits odd quantum effects). In 1994, the NIST achieved a record cold temperature of 700 nK (billionths of a kelvin). In 2003, researchers at MIT eclipsed this with a new record of 450 pK (0.45 nK).
History
To establish an instrument to measure a range of temperatures, in 1593 Galileo Galilei invented a rudimentary water thermometer. One of the first to discuss the possibility of an “absolute cold” on such a scale was Robert Boyle who in his 1665 New Experiments and Observations touching Cold, stated the dispute which is the primum frigidum is very well known among naturalists, some contending for the earth, others for water, others for the air, and some of the moderns for nitre, but all seeming to agree that:
| “ | There is some body or other that is of its own nature supremely cold and by participation of which all other bodies obtain that quality. | ” |
Limit to the 'degree of cold'
The question whether there is a limit to the degree of cold possible, and, if so, where the zero must be placed, was first attacked by the French physicist Guillaume Amontons in 1702, in connection with his improvements in the air thermometer. In his instrument temperatures were indicated by the height at which a column of mercury was sustained by a certain mass of air, the volume or "spring" which of course varied with the heat to which it was exposed. Amontons therefore argued that the zero of his thermometer would be that temperature at which the spring of the air in it was reduced to nothing. On the scale he used the boiling-point of water was marked at +73 and the melting-point of ice at 511, so that the zero of his scale was equivalent to about -240 on the centigrade scale.
This remarkably close approximation to the modern value of -273°C for the zero of the air-thermometer was further improved on by Johann Heinrich Lambert (Pyrometrie, 1779), who gave the value -270°C and observed that this temperature might be regarded as absolute cold.
Values of this order for the absolute zero were not, however, universally accepted about this period. Laplace and Lavoisier, for instance, in their treatise on heat (1780), arrived at values ranging from 1500 to 3000 below the freezing-point of water, and thought that in any case it must be at least 600 below, while John Dalton in his Chemical Philosophy gave ten calculations of this value, and finally adopted -3000°C as the natural zero of temperature.
Lord Kelvin's work
After J. P. Joule had determined the mechanical equivalent of heat, Lord Kelvin approached the question from an entirely different point of view, and in 1848 devised a scale of absolute temperature which was independent of the properties of any particular substance and was based solely on the fundamental laws of thermodynamics. It followed from the principles on which this scale was constructed that its zero was placed at -273°, at almost precisely the same point as the zero of the air-thermometer.
Record temperatures near absolute zero
It can be shown from the laws of thermodynamics that absolute zero can never be achieved artificially, though it is possible to reach temperatures arbitrarily close to it through the use of cryocoolers. This is the same principle that ensures no machine can be 100% efficient.
At very low temperatures in the vicinity of absolute zero, matter exhibits many unusual properties including superconductivity, superfluidity, and Bose-Einstein condensation. In order to study such phenomena, scientists have worked to obtain ever lower temperatures.
- In September 2003, MIT announced a record cold temperature of 450 pK, or 4.5 × 10-10 K in a Bose-Einstein condensate of sodium atoms. This was performed by Wolfgang Ketterle and colleagues at MIT.
- As of February 2003, the Boomerang Nebula, with a temperature of -272.15 Celsius; 1K, is the coldest place known outside a laboratory. The nebula is 5000 light-years from Earth and is in the constellation Centaurus.
- As of November 2000, nuclear spin temperatures below 100 pK were reported for an experiment at the Helsinki University of Technology's Low Temperature Lab. However, this was the temperature of one particular degree of freedom — a quantum property called nuclear spin — not the overall average thermodynamic temperature for all possible degrees of freedom.
Thermodynamics near absolute zero
At temperatures near 0 K, nearly all molecular motion ceases and ΔS = 0 for any adiabatic process. Pure substances can (ideally) form perfect crystals as T 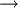 0. Planck's strong form of the third law of thermodynamics states that the entropy of a perfect crystal vanishes at absolute zero. However, if the lowest energy state is degenerate (more than one microstate), this cannot be true. The original Nernst heat theorem makes the weaker and less controversial claim that the entropy change for any isothermal process approaches zero as T → 0
0. Planck's strong form of the third law of thermodynamics states that the entropy of a perfect crystal vanishes at absolute zero. However, if the lowest energy state is degenerate (more than one microstate), this cannot be true. The original Nernst heat theorem makes the weaker and less controversial claim that the entropy change for any isothermal process approaches zero as T → 0
which implies that the entropy of a perfect crystal simply approaches a constant value.
The Nernst postulate identifies the isotherm T = 0 as coincident with the adiabat S = 0, although other isotherms and adiabats are distinct. As no two adiabats intersect, no other adiabat can intersect the T = 0 isotherm. Consequently no adiabatic process initiated at nonzero temperature can lead to zero temperature. (≈ Callen, pp. 189-190)
An even stronger assertion is that It is impossible by any procedure to reduce the temperature of a system to zero in a finite number of operations. (≈ Guggenheim, p. 157)
A perfect crystal is one in which the internal lattice structure extends uninterrupted in all directions. The perfect order can be represented by translational symmetry along three (not usually orthogonal) axes. Every lattice element of the structure is in its proper place, whether it is a single atom or a molecular grouping. For substances which have two (or more) stable crystalline forms, such as diamond and graphite for carbon, there is a kind of "chemical degeneracy". The question remains whether both can have zero entropy at T = 0 even though each is perfectly ordered.
Perfect crystals never occur in practice; imperfections, and even entire amorphous materials, simply get "frozen in" at low temperatures, so transitions to more stable states do not occur.
Using the Debye model, the specific heat and entropy of a pure crystal are proportional to T 3, while the enthalpy and chemical potential are proportional to T 4. (Guggenheim, p. 111) These quantities drop toward their T = 0 limiting values and approach with zero slopes. For the specific heats at least, the limiting value itself is definitely zero, as borne out by experiments to below 10 K. Even the less detailed Einstein model shows this curious drop in specific heats. In fact, all specific heats vanish at absolute zero, not just those of crystals. Likewise for the coefficient of thermal expansion. Maxwell's relations show that various other quantities also vanish. These phenomena were unanticipated.
Since the relation between changes in the Gibbs free energy, the enthalpy and the entropy is
it follows that as T decreases, ΔG and ΔH approach each other (so long as ΔS is bounded). Experimentally, it is found that most chemical reactions are exothermic and release heat in the direction they are found to be going, toward equilbrium. That is, even at room temperature T is low enough so that the fact that (ΔG)T,P < 0 (usually) implies that ΔH < 0. (In the opposite direction, each such reaction would of course absorb heat.)
More than that, the slopes of the temperature derivatives of ΔG and ΔH converge and are equal to zero at T = 0, which ensures that ΔG and ΔH are nearly the same over a considerable range of temperatures, justifying the approximate empirical Principle of Thomsen and Berthelot, which says that the equilibrium state to which a system proceeds is the one which evolves the greatest amount of heat, i.e., an actual process is the most exothermic one. (Callen, pp. 186-187)
Absolute temperature scales
As mentioned, absolute or thermodynamic temperature is conventionally measured in kelvins ( Celsius-size degrees), and increasingly rarely in the Rankine scale ( Fahrenheit-size degrees). Absolute temperature is uniquely determined up to a multiplicative constant which specifies the size of the "degree", so the ratios of two absolute temperatures, T2/T1, are the same in all scales. The most transparent definition comes from the classical Maxwell-Boltzmann distribution over energies, or from the quantum analogs: Fermi-Dirac statistics (particles of half-integer spin) and Bose-Einstein statistics (particles of integer spin), all of which give the relative numbers of particles as (decreasing) exponential functions of energy over kT. On a macroscopic level, a definition can be given in terms of the efficiencies of "reversible" heat engines operating between hotter and colder thermal reservoirs.
Negative temperatures
Certain semi-isolated systems (for example a system of non-interacting spins in a magnetic field) can achieve negative temperatures; however, they are not actually colder than absolute zero. They can be however thought of as "hotter than T=∞", as energy will flow from a negative temperature system to any other system with positive temperature upon contact.