Atom
2007 Schools Wikipedia Selection. Related subjects: General Chemistry
| Atom | ||||||
|---|---|---|---|---|---|---|
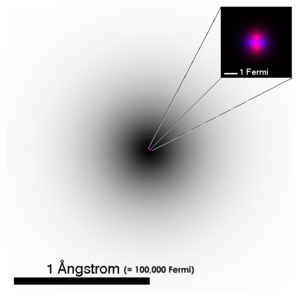
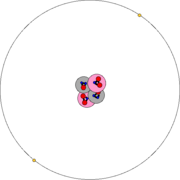
| An accurate depiction of the atomic structure of the helium atom. The darkness of the electron cloud corresponds to the line-of-sight integral over the probability function of the 1s electron orbital. The magnified nucleus is schematic, showing protons in pink and neutrons in purple. In reality, the nucleus (and the wavefunction of each of the nucleons) is also spherically symmetric. (For more complex nuclei this is not the case.) | ||||||
| Classification | ||||||
|
||||||
| Properties | ||||||
|
In chemistry and physics, an atom ( Greek ἄτομος or átomos meaning "indivisible") is the smallest particle of a chemical element that retains its chemical properties. (Since until the advent of quantum mechanics dividing a material object was invariably equated with cutting it, átomos is usually translated as "indivisible".) Where as the word atom originally denoted a particle that cannot be cut into smaller particles, the atoms of modern parlance are composed of subatomic particles:
- electrons, which have a negative charge and are smallest of the three;
- protons, which have a positive charge and are about 1836 times bigger than electrons; and
- neutrons, which have no charge and are about 1839 times bigger than electrons.
Protons and neutrons make up a dense, massive atomic nucleus, and are collectively called nucleons. The electrons form the much larger electron cloud surrounding the nucleus.
Atoms can differ in the number of each of the subatomic particles they contain. Atoms of the same element have the same number of protons (called the atomic number). Within a single element, the number of neutrons may vary, determining the isotope of that element. The number of electrons associated with an atom is most easily changed, due to the lower energy of binding of electrons. The number of protons (and neutrons) in the atomic nucleus may also change, via nuclear fusion, nuclear fission or radioactive decay, in which case the atom is no longer the same element it was.
Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms which have either a deficit or a surplus of electrons are called ions. Electrons that are furthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.
Atoms are the fundamental building blocks of chemistry, and are conserved in chemical reactions.
Atoms and molecules
For gases and certain molecular liquids and solids (such as water and sugar), molecules are the smallest division of matter which retains chemical properties; however, there are also many solids and liquids which are made of atoms, but do not contain discrete molecules (such as salts, rocks, and liquid and solid metals). Thus, while molecules are common on Earth (making up all of the atmosphere and most of the oceans), most of the mass of the Earth (much of the crust, and all of the mantle and core) is not made of identifiable molecules, but rather represents atomic matter in other arrangements, all of which lack the particular type of small-scale order that is associated with molecules.
Most molecules are made up of multiple atoms; for example, a molecule of water is a combination of two hydrogen atoms and one oxygen atom. The term "molecule" in gases has been used as a synonym for the fundamental particles of the gas, whatever their structure. This definition results in a few types of gases (for example inert elements that do not form compounds, such as helium), having "molecules" consisting of only a single atom.
History of atomic theory and discovery of atomic structure
Philosophical atomic ruminations date back to the ancient Greeks and Indians in the fifth and sixth centuries BCE. It was the Greeks ( Democritus; see below) who coined the term atomos, which meant "uncuttable".
The first philosophical statements relating to an idea similar to atoms was developed by Democritus in Greece in the fifth century BCE around 450 BCE. The idea was lost for centuries until scientific interest was rekindled during the Renaissance Period.
In 1803, John Dalton used the concept of atoms to explain why elements always reacted in simple proportions, and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join up to form compound chemicals.
In 1897 JJ Thomson, through his work on cathode rays, discovered the electron and their subatomic nature, which destroyed the concept of atoms as being indivisible units. Thomson would also later discover the existence of isotopes through his work on ionized gases.
Thomson believed that the electrons were distributed evenly throughout the atom, balanced by the presence of a uniform sea of positive charge. However, in 1909, Rutherford's gold foil experiment suggested that the positive charge of an atom and most of its mass was concentrated in a nucleus at the centre of the atom, with the electrons orbiting it like planets around a sun. In 1913, Niels Bohr added quantum mechanics into this model, which now stated that the electrons were confined to clearly defined orbits and could not freely spiral in or out.
In 1926, Erwin Schrodinger proposed that electrons behave not like particles, but like waves. A consequence of this notion, pointed out by Werner Heisenberg a year later, is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at any point in time; this became known as the Uncertainty principle. Instead, for any given value of position you could only obtain a range of probable values for momentum, and vice versa. Thus, the planetary model of the atom was discarded in favour of one that described zones around the nucleus where a given electron is most likely to exist.
Properties of the atom in present theory
Subatomic particles
Although the name "atom" was applied at a time when atoms were thought to be indivisible, it is now known that the atom can be broken down into a number of smaller components. The first of these to be discovered was the negatively charged electron, which is easily ejected from atoms during ionization. The electrons (or more specifically, electron clouds) orbit a small, dense body containing all of the positive charge in the atom, called the atomic nucleus. This nucleus is itself made up of nucleons: positively charged protons and chargeless neutrons.
Before 1961, the subatomic particles were thought to consist of only protons, neutrons and electrons. However, protons and neutrons themselves are now known to consist of still smaller particles called quarks. In addition, the electron is known to have a nearly massless neutral partner called a neutrino. Together, the electron and neutrino are both leptons.
Ordinary atoms are composed only of quarks and leptons of the first generation. The proton is composed of two up quarks and one down quark, whereas the neutron is composed of one up quark and two down quarks. Although they do not occur in ordinary matter, two other heavier generations of quarks and leptons may be generated in high-energy collisions.
The subatomic force carrying particles (called gauge bosons) are also important to atoms. Electrons are bound to the nucleus by photons carrying the electromagnetic force. Protons and neutrons are bound together in the nucleus by gluons carrying the strong nuclear force.
Electron configuration
The nucleus of an atom is surrounded by a cloud of electrons, and it is primarily the interaction of these clouds that govern the chemical behaviour of atoms. A popular concept is that the electrons orbit the atom in neat circles like planets around a sun, but this is an obsolete model that is nonetheless still taught to schoolchildren because it is simpler and sufficient for school-level chemistry. In the true modern model of the atom, the positions of the electrons around the atom's nucleus are described through probabilities—that is, an electron can theoretically be found at any arbitrary position around the nucleus, but is more likely to be found in certain regions than others. This pattern is referred to as its atomic orbital and the shape of its orbital depends on its energy level (or, more specifically, its quantum state).
Each atomic orbital can hold up to two electrons. The orbitals are organized into shells and subshells, based on their overall energy and angular momentum. Generally speaking, higher energy shells can hold more electrons and are located farther from the nucleus. A shell can hold up to 2n2 electrons (where n is the shell number). The electrons in the outermost shell, called the valence electrons, have the greatest influence on chemical behaviour. Core electrons (those not in the outer shell) play a role, but it is usually in terms of a secondary effect due to screening of the positive charge in the atomic nucleus.
In the most stable ground state, an atom's electrons will fill up its orbitals in order of increasing energy. Under some circumstances an electron may be excited to a higher energy level (that is, it absorbs energy from an external source and leaps to a higher shell), leaving a space in a lower shell. An excited atom's electrons will spontaneously fall back to lower levels, emitting the energy it had gained as a photon. This behaviour is the root of a substance's absorption spectrum.
Nucleon properties
The constituent protons and neutrons of the atomic nucleus are collectively called nucleons. The nucleons are held together in the nucleus by the strong nuclear force which is carried by gluons.
Nuclei can undergo transformations that affect the number of protons and neutrons they contain, a process called radioactive decay. When nuclei transformations take place spontaneously, this process is called radioactivity. Radioactive transformations proceed by a wide variety of modes, but the most common are alpha decay (emission of a helium nucleus) and beta decay (emission of an electron). Decays involving electrons or positrons are due to the weak nuclear interaction.
In addition, like the electrons of the atom, the nucleons of nuclei may be pushed into excited states of higher energy. However, these transitions typically require thousands of times more energy than electron excitations. When an excited nucleus emits a photon to return to the ground state, the photon has very high energy and is called a gamma ray.
Nuclear transformations also take place in nuclear reactions. In nuclear fusion, two light nuclei come together and merge into a single heavier nucleus. In nuclear fission, a single large nucleus is divided into two or more smaller nuclei.
Atom size and speed
Atoms are much smaller than the wavelengths of light that human vision can detect, so atoms cannot be seen in any kind of optical microscope. However, there are ways of detecting the positions of atoms on the surface of a solid or a thin film so as to obtain images. These include: electron microscopes (such as in scanning tunneling microscopy (STM)), atomic force microscopy (AFM), nuclear magnetic resonance (NMR) and x-ray microscopy.
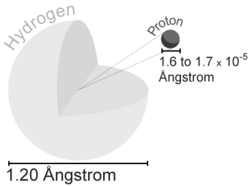
Since the electron cloud does not have a sharp cutoff, the size of an atom is not easily defined. For atoms that can form solid crystal lattices, the distance between the centers of adjacent atoms can be easily determined by x-ray diffraction, giving an estimate of the atoms' size. For any atom, one might use the radius at which the electrons of the valence shell are most likely to be found. As an example, the size of a hydrogen atom is estimated to be approximately 1.06×10-10 m (twice the Bohr radius). Compare this to the size of the proton (the only particle in the nucleus of the hydrogen atom), which is approximately 10-15 m. So the ratio of the size of the hydrogen atom to its nucleus is about 100,000:1. If an atom were the size of a stadium, the nucleus would be the size of a marble. If an atom were the size of the United States, an electron would be 3cm long and wide. Nearly all the mass of an atom is in its nucleus, yet almost all the space in an atom is occupied by its electrons.
Atoms of different elements do vary in size, but the sizes (volumes) do not scale well with the mass of the atom. Heavier atoms do tend generally to be more dense. The diameters of atoms are roughly the same to within a factor of less than three for the heavier atoms, and the most noticeable effect on size with atomic mass is a reverse one: atomic size actually shrinks with increasing mass in each periodic table row . The reason for these effects is that heavy elements have large positive charge on their nuclei, which strongly attract the electrons to the centre of the atom. This contracts the size of the electron shells, so that more electrons may fit into a smaller volume. These effects may be striking: for example, atoms of the densest element iridium (atomic weight about 192) are about the same size as aluminium atoms (atomic weight about 27), and this contributes greatly to the density ratio of more than eight between these metals.
The temperature of a collection of atoms is a measure of the average energy of motion of those atoms above the minimum zero-point energy demanded by quantum mechanics; at 0 kelvins (absolute zero) atoms would have no extra energy above the minimum. As the temperature of the system is increased, the kinetic energy of the particles in the system is increased, and their speed of motion increases. At room temperature, atoms making up gases in the air move at an average speed of 500 m/s (about 1100 mph or 1800 km/h).
Elements, isotopes and ions
Atoms with the same atomic number Z share a wide variety of physical properties and exhibit almost identical chemical properties (for the closest instance to an exception to this principle, see deuterium and heavy water). Atoms are classified into chemical elements by their atomic number Z, which corresponds to the number of protons in the atom. For example, all atoms containing six protons (Z = 6) are classified as carbon. The elements may be sorted according to the periodic table in order of increasing atomic number. When this is done, certain repeating cycles of regularities in chemical and physical properties are evident.
The mass number A, or nucleon number of an element is the total number of protons and neutrons in an atom of that element, so-called because each proton and neutron has a mass of about 1 amu. A particular collection of a certain number of protons Z, and neutrons A-Z, is called a nuclide.
Each element can have numerous different nuclides with the same Z (number of protons and electrons) but varying numbers of neutrons. Such a family of nuclides are called the isotopes of the element (isotope = "same place", because these nuclides share the same chemical symbol and place on the periodic table). When writing the name of a particular nuclide, the element name (which specifies the Z) is preceded by the mass number if written as a superscript, or else followed by the mass number if not a superscript. For example, the nuclide carbon-14, which may also be written 14C, is one of the isotopes of carbon, and it contains 6 protons and 8 neutrons in each atom, for a total mass number of 14. For a complete table of known nuclides, including radioactive and stable nuclides, see isotope table (divided).
The atomic mass listed for each element in the periodic table is an average of the isotope masses found in nature, weighted by their abundance.
The simplest atom is the hydrogen isotope protium, which has atomic number 1 and atomic mass number 1; it consists of one proton and one electron. The hydrogen isotope which also contains one neutron so is called deuterium or hydrogen-2; the hydrogen isotope with two neutrons is called tritium or hydrogen-3. Tritium is an unstable isotope which decays through a process called radioactivity. Many isotopes of each element are radioactive; the number which are stable varies greatly with the element (tin has 10 stable isotopes; see list of stable isotopes). Lead (Z = 82) is the last element which has stable isotopes. The elements with atomic number 83 (bismuth) and greater have no stable isotopes and are all radioactive.
Virtually all elements heavier than hydrogen and helium were created through stellar nucleosynthesis and supernova nucleosynthesis. The solar system is thought to be formed of clouds of elements from many such supernovae, which date from more than 4.6 billion years ago. Most of the elements lighter than uranium (Z = 92) have either stable isotopes, or else radioisotopes long-lived enough to occur naturally on Earth. Two notable exceptions of light but short-lived radioactive elements are technetium Z = 43 (although some technetium has been found on Earth, this occurred only after the element was first synthesized artificially), and promethium Z = 61, which is found naturally only in stars where it was recently made. Several other short-lived heavy elements that do not occur on Earth have been found to be present in stars. Elements not normally found in nature have been artificially created by nuclear bombardment; as of 2006, elements have been created through atomic number 116 (given the temporary name ununhexium). These ultra-heavy elements are generally highly unstable and decay quickly.
Atoms that have lost or gained electrons to become electrically non-neutral, are called atomic ions. Ions are divided into cations with positive (+) electric charge; or anions with negative (-) charge.
Valence and bonding
The number of electrons in an atom's outermost shell (the valence shell) governs its bonding behaviour. Therefore, elements with the same number of valence electrons are grouped together in the columns of the periodic table of the elements. Alkali metals contain one electron on their outer shell; alkaline earth metals, two electrons; halogens, seven electrons; and various others.
Every atom is most stable with a full valence shell. This means that atoms with full valence shells (the noble gases) are very unreactive. Conversely, atoms with few electrons in their valence shell are more reactive. Alkali metals are therefore very reactive, with caesium, rubidium, and francium being the most reactive of all metals. Also, atoms that need only few electrons (such as the halogens) to fill their valence shells are reactive. Fluorine is the most reactive of all elements.
Atoms may fill their valence shells by chemical bonding. This can be achieved one of two ways: an atom can either share electrons with other atoms (a covalent bond), or it can remove electrons from (or donate electrons to) other atoms (an ionic bond). The formation of a bond causes a strong attraction between two atoms, creating molecules or ionic compounds. Many other types of bonds exist, including:
- polar covalent bonds;
- coordinate covalent bonds;
- metallic bonds;
- hydrogen bonds; and
- van der Waals bonds.
Atomic spectrum
Since each element in the periodic table consists of an atom in a unique configuration with different numbers of protons and electrons, each element can also be uniquely described by the energies of its atomic orbitals and the number of electrons within them. Normally, an atom is found in its lowest-energy ground state; states with higher energy are called excited states. An electron may move from a lower-energy orbital to a higher-energy orbital by absorbing a photon with energy equal to the difference between the energies of the two levels. An electron in a higher-energy orbital may drop to a lower-energy orbital by emitting a photon. Since each element has a unique set of energy levels, each creates its own light pattern unique to itself: its own spectral signature.
If a set of atoms is heated (such as in an arc lamp), their electrons will move into excited states. When these atoms fall back toward the ground state, they will produce an emission spectrum. If a set of atoms is illuminated by a continuous spectrum, it will only absorb specific wavelengths (energies) of photon that correspond to the differences in its energy levels. The resulting pattern of gaps is called the absorption spectrum.
In spectroscopic analysis, scientists can use a spectrometer to study the atoms in stars and other distant objects. Due to the distinctive spectral lines that each element produces, they are able to tell the chemical composition of distant planets, stars and nebulae.
Not all parts of the atomic spectrum are in visible light part of the electromagnetic spectrum. For example, the hyperfine transitions (including the important 21 cm line) produce low-energy radio waves. When electrons deep inside atoms of high atomic number are knocked out (for example by beta radiation), replacement electrons fall deep into the electric potential of the high-Z nucleus, producing high-energy x-rays.
Exotic atoms
An exotic atom is usually made from a normal matter atom with a substitution from abnormal or rarely encountered matter, such as antimatter, muons, mesons, or other objects. A few exotic atoms (such as atoms of antimatter) are not made of any normal atomic constituents at all. All exotic atoms (save antiatoms made from antinucleons and positrons), are highly unstable, decaying with lifetimes of a few microseconds or less. The antimatter counterparts of stable particles are also stable, but difficult to store for more than short periods, since they annihilate if allowed to contact ordinary matter.
The most familiar examples of exotic atoms are the antiatom antihydrogen (composed of an antiproton and positron) which has been produced in tiny quantities, and positronium, an analogue to the hydrogen atom in which a positron is substituted for the usual proton nucleus. Positronium is unstable; it is a common phase in the attraction between an electron and positron before the annihilation reaction in which the matter particles are destroyed and two gamma rays are emitted.
Atoms and the Big Bang Theory
In models of the Big Bang, Big Bang nucleosynthesis predicts that within one to three minutes of the Big Bang almost all atomic material in the universe was created. During this process, nuclei of hydrogen and helium formed abundantly, but almost no elements heavier than lithium. Hydrogen makes up approximately 75% of the atoms in the universe; helium makes up 24%; and all other elements make up just 1%. However, although nuclei (fully- ionized atoms) were created, neutral atoms themselves could not form in the intense heat.
Big Bang chronology of the atom continues to approximately 379,000 years after the Big Bang when the cosmic temperature had dropped to just 3,000 K. It was then cool enough to allow the nuclei to capture electrons. This process is called recombination, during which the first neutral atoms took form. Once atoms become neutral, they only absorb photons of a discrete absorption spectrum. This allows most of the photons in the universe to travel unimpeded for billions of years. These photons are still detectable today in the cosmic microwave background.
After Big Bang nucleosynthesis, no heavier elements could be created until the formation of the first stars. These stars fused heavier elements through stellar nucleosynthesis during their lives and through supernova nucleosynthesis as they died. The seeding of the interstellar medium by heavy elements eventually allowed the formation of terrestrial planets like the Earth.
Atom size comparisons
Various analogies have been used to demonstrate the minuteness of the atom:
- A human hair is about 1 million carbon atoms wide.
- An HIV virus is the width of 800 carbon atoms and contains about 100 million atoms total. An E. coli bacterium contains perhaps 100 billion atoms.
- A speck of dust might contain 3x1012 (3 trillion) atoms.
- The number of atoms in 12 grams of charcoal (about 6 x 1023) is more than 1,400,000 times the age of the universe in seconds.