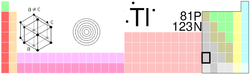
Thallium
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | thallium, Tl, 81 | |||||||||||||||||||||||||||
| Chemical series | poor metals | |||||||||||||||||||||||||||
| Group, Period, Block | 13, 6, p | |||||||||||||||||||||||||||
| Appearance | silvery white |
|||||||||||||||||||||||||||
| Atomic mass | 204.3833 (2) g/mol | |||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p1 | |||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 3 | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||
| Density (near r.t.) | 11.85 g·cm−3 | |||||||||||||||||||||||||||
| Liquid density at m.p. | 11.22 g·cm−3 | |||||||||||||||||||||||||||
| Melting point | 577 K (304 ° C, 579 ° F) |
|||||||||||||||||||||||||||
| Boiling point | 1746 K (1473 ° C, 2683 ° F) |
|||||||||||||||||||||||||||
| Heat of fusion | 4.14 kJ·mol−1 | |||||||||||||||||||||||||||
| Heat of vaporization | 165 kJ·mol−1 | |||||||||||||||||||||||||||
| Heat capacity | (25 °C) 26.32 J·mol−1·K−1 | |||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||
| Oxidation states | 3, 1 (mildly basic oxide) |
|||||||||||||||||||||||||||
| Electronegativity | 1.62 (Pauling scale) | |||||||||||||||||||||||||||
| Ionization energies | 1st: 589.4 kJ/mol | |||||||||||||||||||||||||||
| 2nd: 1971 kJ/mol | ||||||||||||||||||||||||||||
| 3rd: 2878 kJ/mol | ||||||||||||||||||||||||||||
| Atomic radius | 190 pm | |||||||||||||||||||||||||||
| Atomic radius (calc.) | 156 pm | |||||||||||||||||||||||||||
| Covalent radius | 148 pm | |||||||||||||||||||||||||||
| Van der Waals radius | 196 pm | |||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||
| Magnetic ordering | ??? | |||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 0.18 µΩ·m | |||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 46.1 W·m−1·K−1 | |||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 29.9 µm·m−1·K−1 | |||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 818 m/s | |||||||||||||||||||||||||||
| Young's modulus | 8 GPa | |||||||||||||||||||||||||||
| Shear modulus | 2.8 GPa | |||||||||||||||||||||||||||
| Bulk modulus | 43 GPa | |||||||||||||||||||||||||||
| Poisson ratio | 0.45 | |||||||||||||||||||||||||||
| Mohs hardness | 1.2 | |||||||||||||||||||||||||||
| Brinell hardness | 26.4 MPa | |||||||||||||||||||||||||||
| CAS registry number | 7440-28-0 | |||||||||||||||||||||||||||
| Selected isotopes | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||
Thallium ( IPA: /ˈθaliəm/) is a chemical element in the periodic table that has the symbol Tl and atomic number 81. This soft gray malleable poor metal resembles tin but discolors when exposed to air. Thallium is highly toxic and is used in rat poisons and insecticides but since it might also cause cancer (although the EPA does not class it as carcinogen), this use has been cut back or eliminated in many countries. It is also used in infrared detectors. It has even been used in some murders, earning the nicknames "The Poisoner's Poison" and "Inheritance powder" (alongside arsenic).
Notable characteristics
This metal is very soft and malleable and can be cut with a knife. When it is first exposed to air, thallium has a metallic luster but quickly tarnishes with a bluish-gray tinge that resembles lead (it is preserved by keeping it under oil). A heavy layer of oxide builds up on thallium if left in air. In the presence of water, thallium hydroxide is formed.
Applications
The odorless and tasteless thallium sulfate was widely used in the past as a rat poison and ant killer. In the United States and many other countries this use is no longer allowed due to safety concerns. Other uses:
- thallium sulfide's electrical conductivity changes with exposure to infrared light therefore making this compound useful in photocells.
- thallium bromide- iodide crystals have been used as infrared optical materials.
- thallium oxide has been used to manufacture glasses that have a high index of refraction.
- used in semiconductor materials for selenium rectifiers,
- in gamma radiation detection equipment,
- high-density liquid used for sink-float separation of minerals,
- used in the treatment of ringworm and other skin infections. However this use has been limited due to the narrow margin that exists between toxicity and therapeutic benefit.
- radioactive thallium-201 (half-life of 73 hours) is used for diagnostic purposes in nuclear medicine, particularly in stress tests used for risk stratification in patients with coronary artery disease (CAD). . This isotope of thallium can be generated using a transportable generator which is similar to the technetium cow. The generator contains lead-201 (half life 9.33 hours) which decays by electron capture to the thallium-201. The lead-201 can be produced in a cyclotron by the bombardment of thallium with with protons or deuterons by the (p,3n) and (d,4n) reactions.
- combined with sulfur or selenium and arsenic, thallium has been used in the production of high- density glasses that have low melting points in the range of 125 and 150 °C. These glasses have room temperature properties that are similar to ordinary glasses and are durable, insoluble in water and have unique refractive indices.
- thallium amalgam is used in thermometers for low temperature, because it freezes at -58 °C (pure mercury freezes at -38 °C).
In addition, research activity with thallium is ongoing to develop high-temperature superconducting materials for such applications as magnetic resonance imaging, storage of magnetic energy, magnetic propulsion, and electric power generation and transmission.
History
Thallium ( Greek θαλλός, thallos, meaning "a green shoot or twig") was discovered by Sir William Crookes in 1861 in England while he was making spectroscopic determinations for tellurium on residues from a sulfuric acid plant. The name comes from Thallium's bright green spectral emission lines. In 1862 Crookes and Claude-Auguste Lamy isolated the metal independently of each other.
Occurrence
Although the metal is reasonably abundant in the Earth's crust at a concentration estimated to be about 0.7 mg/kg, mostly in association with potassium minerals in clays, soils, and granites, it is not generally considered to be commercially recoverable from those forms. The major source of commercial thallium is the trace amounts found in copper, lead, zinc, and other sulfide ores.
Thallium is found in the minerals crookesite TlCu7Se4, hutchinsonite TlPbAs5S9, and lorandite TlAsS2. This metal is also contained in pyrites and is extracted as a by-product of sulfuric acid production when pyrite ore is roasted. Another way this element is obtained is from the smelting of lead and zinc rich ores. Manganese nodules found on the ocean floor also contain thallium, but nodule extraction is prohibitively expensive and potentially environmentally destructive. In addition, several other thallium minerals, containing 16% to 60% thallium, occur in nature as sulfide or selenide complexes with antimony, arsenic, copper, lead, and silver but are rare and have no commercial importance as sources of this element. See also: Category:Thallium minerals.
Isotopes
Thallium has 25 isotopes which have atomic masses that range from 184 to 210. 203Tl and 205Tl are the only stable isotopes and 204Tl is the most stable radioisotope with a half-life of 3.78 years.
Thallium-202 (half life 12.23 days) can be made in a cyclotron while thallium-204 (half life 3.78 years) is made by the neutron activation of stable thallium in a nuclear reactor.
Toxicity
Thallium and its compounds are highly toxic and should be handled with great care. Contact with skin is dangerous and adequate ventilation should be provided when melting this metal. Thallium(I) compounds have a high aqueous solubility and are readily absorbed through the skin. Exposure to them should not exceed 0.1 mg per m² of skin in an 8-hour time-weighted average (40-hour work week). Thallium is a suspected human carcinogen.
Part of the reason for thallium's high toxicity is that, when present in aqueous solution as the univalent thallium(I) ion (Tl+), it exhibits some similarities with essential alkali metal cations, particularly potassium. It can thus enter the body via potassium uptake pathways. However other aspects of thallium's chemistry are very different from that of the alkali metals (e.g. its high affinity for sulfur ligands), and so this substitution disrupts many cellular processes (for instance thallium may attack sulphur-containing proteins such as cysteine residues and ferredoxins).
Thallium's toxicity has led to its use (now discontinued in many countries) as a rat and ant poison.
Amongst the distinctive effects of thallium poisoning are loss of hair (which ironically led it to its initial use as a depilatory, before its toxicity was properly appreciated), and damage to peripheral nerves (victims may experience a sensation of walking on hot coals). Thallium was once an effective murder weapon before its effects became understood and an antidote ( prussian blue) discovered.
Treatment and internal decontamination
One of the main methods of removing thallium (both radioactive and normal) from humans is to use Prussian blue, which is a solid ion exchange material which absorbs thallium and releases potassium. The prussian blue is fed by mouth to the person, and it passes through their digestive system and comes out in the stool.
Famous uses as a poison
- Agatha Christie, who worked as a pharmacist, used thallium as the agent of murder in her detective fiction novel The Pale Horse — the first clue to the murder method coming from the hair loss of the victims.
- The CIA is believed (by its Inspector General) to have conceived a scheme to poison Fidel Castro by exposure to thallium salts placed in his shoes while they were being polished. The goal was to discredit him by causing him to lose his characteristic hair and beard. The scheme progressed as far as testing on animals, but the trip during which the poison was to be administered fell through.
- In 1953, Australian Caroline Grills was sentenced to life in prison after three family members and a close family friend died. Authorities found thallium in tea that she had given to two additional family members.
- Dr. Félix-Roland Moumié, a leader of the Cameroonian anticolonial armed struggle against France was murdered by thallium poisoning on October 15, 1960. A French agent posing as a journalist was the main suspect of this murder.
- It is claimed that South African agents once plotted to use it against Nelson Mandela while he was in prison on Robben Island. The Truth and Reconciliation Commission heard how agents established plans to add doses of the chemical to his medication.
- In the 1960s and 1970s, Graham Frederick Young killed at least three people with thallium. The 1995 film The Young Poisoner's Handbook is based upon him.
- In 1995, Zhu Ling, a student at Tsinghua University in Beijing, China, was reportedly poisoned twice by her roommate, over a period of a few months. The classmates of the victim asked for help through Usenet, to which access was very new in mainland China at the time. Joint efforts by physicians who responded through the web led to the diagnosis of thallium poisoning. The case was covered by news reports around the world.
- In June 2004, 25 Russian soldiers earned Honorable Mention Darwin Awards after becoming ill from thallium exposure when they found a can of mysterious white powder in a rubbish dump on their base at Khabarovsk in the Russian Far East. Oblivious to the danger of misusing an unidentified white powder from a military dump site, the conscripts added it to tobacco, and used it as a substitute for talcum powder on their feet.
- In 2005, a 17 year old girl in Numazu, Shizuoka, Japan, admitted to attempting to murder her mother by lacing her tea with thallium, causing a national scandal.