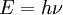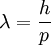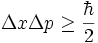Wave–particle duality
2007 Schools Wikipedia Selection. Related subjects: General Physics
In physics and chemistry, wave-particle duality holds that light and matter exhibit properties of both waves and of particles. A central concept of quantum mechanics, duality represents a way to address the inadequacy of conventional concepts like "particle" and "wave" to meaningfully describe the behaviour of quantum objects. The idea of duality is rooted in a debate over the nature of light and matter dating back to the 1600s, when competing theories of light were proposed by Christiaan Huygens and Isaac Newton. Through the work of Albert Einstein, Louis de Broglie and many others, it is now established that all objects have both wave and particle nature (though this phenomenon is only detectable on small scales, such as with atoms), and that a suitable interpretation of quantum mechanics provides the over-arching theory resolving this ostensible paradox.
History
At the close of the 19th century, the case for atomic theory, that matter was made of particulate objects or atoms, was well established. Electricity, first thought to be a fluid, was understood to consist of particles called electrons, as demonstrated by J.J. Thomson by his research into the work of Rutherford, who had investigated using cathode rays that an electrical charge would actually travel across a vacuum from cathode to anode. In brief, it was understood that much of nature was made of particles. At the same time, waves were well understood, together with wave phenomena such as diffraction and interference. Light was believed to be a wave, as Thomas Young's double-slit experiment and effects such as Fraunhofer diffraction had clearly demonstrated the wave-like nature of light.
But as the 20th century turned, problems had emerged with this viewpoint. The photoelectric effect, as analyzed in 1905 by Albert Einstein, demonstrated that light also possessed particle-like properties, further confirmed with the discovery of the Compton effect in 1923. Later on, the diffraction of electrons would be predicted and experimentally confirmed, thus showing that electrons must have wave-like properties in addition to particle properties.
This confusion over particle versus wave properties was eventually resolved with the advent and establishment of quantum mechanics in the first half of the 20th century, which ultimately explained wave-particle duality. It provided a single unified theoretical framework for understanding that all matter may have characteristics associated with particles and waves. Quantum mechanics holds that every particle in nature, be it a photon, electron or atom, is described by a solution to a differential equation, most typically, the Schroedinger equation. The solutions to this equation are known as wave functions, as they are inherently wave-like in their form. They can diffract and interfere, leading to the wave-like phenomena that are observed. Yet also, the wave functions are interpreted as describing the probability of finding a particle at a given point in space. Thus, if one is looking for a particle, one will find one, with a probability density given by the square of the magnitude of the wave function.
One does not observe the wave-like quality of everyday objects because the associated wavelengths of people-sized objects are exceedingly small.
Huygens and Newton; earliest theories of light
The earliest comprehensive theory of light was advanced by Christiaan Huygens, who proposed a wave theory of light, and in particular demonstrated how waves might interfere to form a wave-front, propagating in a straight line. However, the theory had difficulties in other matters, and was soon overshadowed by Isaac Newton's corpuscular theory of light. That is, Newton proposed that light consisted of small particles, with which he could easily explain the phenomenon of reflection. With considerably more difficulty, he could also explain refraction through a lens, and the splitting of sunlight into a rainbow by a prism.
Because of Newton's immense intellectual stature, his theory went essentially unchallenged for over a century, with Huygens' theories all but forgotten. With the discovery of diffraction in the early 19th century, the wave theory was revived, and so by the advent of the 20th century, a scientific debate over waves vs. particles had already been thriving for a very long time.
Fresnel, Maxwell, and Young
In the early 1800s, the double-slit experiments by Young and Fresnel provided evidence for Huygens' theories: these experiments showed that when light is sent through a grid, a characteristic interference pattern is observed, very similar to the pattern resulting from the interference of water waves; the wavelength of light can be computed from such patterns. Maxwell, during the late- 1800s, explained light as the propagation of electromagnetic waves with the Maxwell equations. These equations were verified by experiment, and Huygens' view became widely accepted.
Einstein and photons
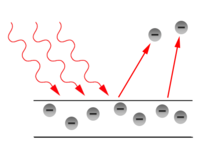
In 1905, Albert Einstein provided an explanation of the photoelectric effect, a hitherto troubling experiment which the wave theory of light seemed incapable of explaining. He did so by postulating the existence of photons, quanta of light energy with particulate qualities.
In the photoelectric effect, it was observed that shining a light on certain metals would lead to an electric current in a circuit. Presumably, the light was knocking electrons out of the metal, causing them to flow. However, it was also observed that while a dim blue light was enough to cause a current, even the strongest, brightest red light caused no current at all. According to wave theory, the strength or amplitude of a light wave was in proportion to its brightness: a bright light should have been easily strong enough to create a large current. Yet, oddly, this was not so.
Einstein explained this conundrum by postulating that the electrons were knocked free of the metal by incident photons, with each photon carrying an amount of energy E that was related to the frequency, ν of the light by
where h is Planck's constant (6.626 x 10-34 J seconds). Only photons of a high-enough frequency, (above a certain threshold value) could knock an electron free. For example, photons of blue light had sufficient energy to free an electron from the metal, but photons of red light did not. More intense light above the threshold frequency could release more electrons, but no amount of light below the threshold frequency could release an electron.
Einstein was awarded the Nobel Prize in Physics in 1921 for his theory of the photoelectric effect.
de Broglie
In 1924, Louis-Victor de Broglie formulated the de Broglie hypothesis, claiming that all matter, not just light, has a wave-like nature; he related wavelength, λ (lambda), and momentum, p:
This is a generalization of Einstein's equation above since the momentum of a photon is given by p = E / c where c is the speed of light in vacuum, and λ = c / ν.
de Broglie's formula was confirmed three years later for electrons (which have a rest-mass) with the observation of electron diffraction in two independent experiments. At the University of Aberdeen, George Paget Thomson passed a beam of electrons through a thin metal film and observed the predicted interference patterns. At Bell Labs Clinton Joseph Davisson and Lester Halbert Germer guided their beam through a crystalline grid.
de Broglie was awarded the Nobel Prize for Physics in 1929 for his hypothesis. Thomson and Davisson shared the Nobel Prize for Physics in 1937 for their experimental work.
Heisenberg
Wave-particle duality is often expressed via the Heisenberg uncertainty principle, which states, in its most common form, that:
where
- Δ is a measure of uncertainty or imprecision in the measurement.
- x and p are a particle's position and linear momentum respectively.
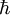 is the reduced Planck's constant (Planck's constant divided by 2π).
is the reduced Planck's constant (Planck's constant divided by 2π). - x and p are a particle's position and linear momentum respectively.
However the relationship holds more generally for any two conjugate variables, such as time and energy, or angle of rotation and angular momentum and follows from the de Broglie hypothesis being applied to classical fields. The uncertainty relation implies that the measurement of one variable results in the disturbance of its conjugate partner, so that the product of their uncertainty is always greater than a certain amount.
Wave behaviour of large objects
Similar experiments have since been conducted with neutrons and protons. Among the most famous experiments are those of Estermann and Otto Stern in 1929. Authors of similar recent experiments with atoms and molecules claim that these larger particles also act like waves.
A dramatic series of experiments emphasizing the action of gravity in relation to wave-particle duality were conducted in the 1970's using the neutron interferometer. Neutrons, one of the components of the atomic nucleus, provide much of the mass of a nucleus and thus of ordinary matter. Neutrons are fermions, and thus possess an important quality we associate with matter, namely its "rigidness" (due to the fact that they obey the Pauli Exclusion Principle). In the neutron interferometer, they act as quantum-mechanical waves directly subject to the force of gravity. While the results were not surprising since gravity was known to act on everything - even deflecting light and acting on photons (the Pound-Rebka falling photon experiment), the self-interference of the quantum mechanical wave of a massive fermion in a gravitational field had never been experimentally confirmed before.
In 1999, the diffraction of C60 fullerenes by researchers from the University of Vienna was reported. Fullerenes are rather large and massive objects, having an atomic mass of about 720. The de Broglie wavelength is 2.5 picometers, whereas the diameter of the molecule is about 1 nanometer, i.e. about 400 times larger. As of 2005, this is the largest object for which quantum-mechanical wave-like properties have been directly observed in far-field diffraction. The experimenters have assumed the arguments of wave-particle duality and have assumed the validity of de Broglie's equation in their argument. In 2003 the Vienna group has meanwhile also demonstrated the wave-nature of tetraphenylporphyrin - a flat biodye with an extension of about 2 nm and a mass of 614 amu. For this demonstration they employed a near-field Talbot Lau interferometer . In the same interferometer they also found interference fringes for C60F48, a fluorinated buckyball with a mass of about 1600 amu, composed of 108 atoms . Large molecules are already so complex that they give experimental access to some aspects of the quantum-classical interface, i.e. to certain decoherence mechanisms .
Whether objects heavier than the Planck mass (about the weight of a large bacterium) have a de Broglie wavelength is theoretically unclear and experimentally unreachable; above the Planck mass a particle's Compton wavelength would be smaller than the Planck length and its own Schwarzchild radius, a scale at which current theories of physics may break down or need to be replaced by more general ones.
Theoretical sketch and remarks on philosophical inquiry
Examples ( diffraction, interference, double-slit experiment, phase noise in lasers, proton, teleportation, quantum computing, quantum cryptography , Bell's theorem, combination with special relativity: Klein-Gordon Equation and Dirac equations) for the application of this framework have been given above, now the common mathematics behind it is discussed.
The wave and the particle description is made equivalent three steps:
- For particles the state of a system is described mathematically by the number of particles and their positions; quantum mechanics assigns every number and every combination of positions a complex number.
- For waves the state of a system is described mathematically by the field distribution; quantum field theory assigns every field distribution (zero everywhere, homogeneous , circular ...) a complex number.
- It is mathematically proven (see fock space in quantum field theory), that both quantum descriptions are equivalent, while the classical descriptions are not
see also: Hilbert space, path integral formulation, Mathematical formulation of quantum mechanics
All complex numbers together make up the ket, which is also called wave function. But the name "wave function" is problematic, as it sounds that they have more to do with waves than with particles. The time evolution of a ket is governed by a partial differential equation generically called the Schrödinger equation. The mathematical tools learned for classical physics to solve such an equation can still be applied: Superposition, eigenfunctions, eigenvalues, FDTD, perturbation theory. In other words: "it is a particle and a wave at the same time" in reality and in calculations. It has to be admitted that the coefficients for the differential equations were derived in a rather unsymmetrical way with respect to particles and waves, but it is unclear if this is an historical artifact.
The ket is still interpreted as the probability of finding a system in a specific state. Most of the examples allow or even need partial measurements followed by a second time evolution of the ket followed by more measurements and so on, this is where the philosophical inquiry takes place.
Applications
Wave-particle duality is exploited in electron microscopy, where the small wavelengths associated with the electron can be used to view objects much smaller than what is visible using visible light.