Platinum
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
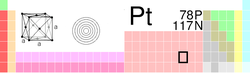
| Name, Symbol, Number | platinum, Pt, 78 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 10, 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | grayish white |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 195.084 (9) g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d9 6s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 17, 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 21.45 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 19.77 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2041.4 K (1768.3 ° C, 3214.9 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4098 K (3825 ° C, 6917 ° F) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 22.17 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 469 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 25.86 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic face centered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 3, 4 (mildly basic oxide) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.28 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 870 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1791 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 135 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 177 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 128 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 175 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 105 nΩ·m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 71.6 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 8.8 µm·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | ( r.t.) 2800 m·s−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 168 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 61 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 230 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 549 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 392 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-06-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Platinum ( IPA: /ˈplætɪnəm/) is a chemical element in the periodic table that has the symbol Pt and atomic number 78. A heavy, malleable, ductile, precious, grey-white transition metal, platinum is resistant to corrosion and occurs in some nickel and copper ores along with some native deposits. Platinum is used in jewelry, laboratory equipment, electrical contacts, dentistry, and automobile emissions control devices.
Notable characteristics
When pure the metal appears greyish-white and firm. The metal is corrosion-resistant. The catalytic properties of the six platinum family metals are outstanding. For this catalytic property, platinum is used in catalytic converters, incorporated in automobile exhaust systems, as well as tips of spark plugs.
Platinum's wear- and tarnish-resistance characteristics are well suited for making fine jewelry. Platinum is more precious than gold. The price of platinum changes along with its availability, but it normally costs slightly less than twice the price of gold. In the 18th century, platinum's rarity made King Louis XV of France declare it the only metal fit for a king.
Platinum possesses remarkable resistance to chemical attack, excellent high-temperature characteristics, and stable electrical properties. All these properties have been exploited for industrial applications. Platinum does not oxidize in air at any temperature, but can be corroded by cyanides, halogens, sulfur, and caustic alkalis. This metal is insoluble in hydrochloric and nitric acid, but does dissolve in the mixture known as aqua regia (forming chloroplatinic acid). Common oxidation states of platinum include +2, +3, and +4.
Applications
- As a catalyst in the catalytic converter, an optional component of the gasoline-fueled automobile exhaust system (see "Notable characteristics" in this article).
- As a catalyst in fuel cells. Reducing the amount of platinum required (and thus cost) is a major focus of fuel cell research.
- Certain platinum-containing compounds are capable of crosslinking (or alkylating) with DNA and are chemotherapeutic agents owing to this capability. For example, cisplatin, Carboplatin and oxaliplatin belong to this class of drugs.
- Platinum resistance thermometers.
- Electrodes for use in electrolysis.
- In the Clark polarographic electrode for measuring oxygen tension.
- A wide range of jewellery
- As a catalyst in the curing of silicone elastomers.
History
Naturally-occurring platinum and platinum-rich alloys have been known for a long time. Though the metal was used by pre-Columbian Native Americans, the first European reference to platinum appears in 1557 in the writings of the Italian humanist Julius Caesar Scaliger (1484-1558) as a description of a mysterious metal found in Central American mines between Darién (Panama) and Mexico ("up until now impossible to melt by any of the Spanish arts").
Platinum was discussed by astronomer Antonio de Ulloa and Don Jorge Juan y Santacilia (1713-1773), both appointed by King Philip V to join a geographical expedition in Peru that lasted from 1735 to 1745. Among other things, Ulloa observed the platina del pinto, the unworkable metal found with gold in New Granada (Colombia). British privateers intercepted Ulloa's ship on the return voyage. Though he was well-treated in England, and even made a member of the Royal Society he was prevented from publishing a reference to the unknown metal until 1748. Before that could happen Charles Wood independently isolated the element in 1741.
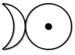
The alchemical symbol for platinum (shown below) was made by joining the symbols of silver and gold.
Occurrence
Platinum is an extremely rare metal, occurring as only 5 ppb in the Earth's crust.
Platinum is often found chemically uncombined as native platinum and alloyed with iridium as platiniridium. The platinum arsenide, sperrylite (PtAs2), is a major source of platinum associated with nickel ores in the Sudbury Basin deposit in Ontario, Canada. The rare sulfide mineral cooperite, (Pt,Pd,Ni)S, contains platinum along with palladium and nickel. Cooperite occurs in the Merensky Reef within the Bushveld complex, Transvaal, South Africa. South Africa is the largest producer of platinum in the world.
Platinum, often accompanied by small amounts of other platinum family metals, occurs in alluvial placer deposits in the Witwatersrand of South Africa, Colombia, Ontario, the Ural Mountains, and in certain western American states.
Platinum is produced commercially as a by-product of nickel ore processing in the Sudbury deposit. The huge quantities of nickel ore processed makes up for the fact that platinum is present as only 0.5 ppm in the ore.
Isotopes
Naturally occurring platinum is composed of five stable isotopes and one radioisotope, Pt-190, which has a very long half-life (over 6 billion years or 190 Ps). There are also many other radioisotopes with the most stable being Pt-193 with a half-life of 50 years.
Precautions
According to the Centers for Disease Control and Prevention, short-term exposure to "platinum salts may cause irritation of the eyes, nose, and throat" and long-term exposure "may cause both respiratory and skin allergies." The current OSHA standard is 0.002 milligram per cubic meter of air averaged over an 8-hour work shift.
Certain platinum complexes (cis-platin) have been used in chemotherapy, as they have very good anti-tumor activity, particularly when used to combat testicular cancer, although they also cause cumulative, irreversible kidney damage.
As platinum is a catalyst in the manufacture of the silicone rubber and gel components of several types of medical implants (breast implants, joint replacement prosthetics, artificial lumbar discs, vascular access ports), the possibility that platinum free radicals could enter the body and cause adverse effects has merited study. However, the FDA has reviewed the issue and found no evidence to suggest toxicity in vivo. .
Rarity and colour
Platinum's rarity as a metal has caused advertisers to associate it with exclusivity and wealth. "Platinum" credit cards have greater privileges than do "gold" ones. "Platinum awards" are the highest possible, ranking above gold, silver and bronze. For example, a musical album that has sold more than 1,000,000 copies, will be credited as "platinum" (a higher certification of " Diamond" does exist, however). And some products, such as blenders and vehicles, with a silvery-white colour are identified as "platinum". Platinum is considered a precious metal, although its use as such is not as common as the use of gold or silver. The frame of the Crown of Queen Elizabeth the Queen Mother, manufactured for her Coronation as Consort of King George VI, is made of platinum. It was the first British crown to be made of that metal. Due to its rarity, platinum is a highly priced metal, more so than gold or silver.
World production
World supply of platinum is around 6 million troy ounces (190,000 kg) per year. Platinum's cost fluctuates around USD $1100 per ounce.