Action potential
2007 Schools Wikipedia Selection. Related subjects: General Biology
An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal life, rapidly carrying information within and between tissues. They are also exhibited by some plants. Action potentials can be created by many types of cells, but are used most extensively by the nervous system for communication between neurons and to transmit information from neurons to other body tissues such as muscles and glands.
Action potentials are not the same in all cell types and can even vary in their properties at different locations in the same cell. For example, cardiac action potentials are significantly different from the action potentials in most neurons. This article is particularly concerned with the "typical" action potential of axons.
Overview
A voltage, or difference in electrostatic potential, always exists between the inside and outside of a cell. This results from the distribution of ions across the cell membrane and from the permeability of the membrane to these ions. The voltage of an inactive cell stays at a negative value (inside relative to outside the cell) and varies little. When the membrane of an excitable cell is depolarized beyond a threshold, the cell will undergo (or "fire") an action potential, often called a "spike" (see Threshold and initiation).
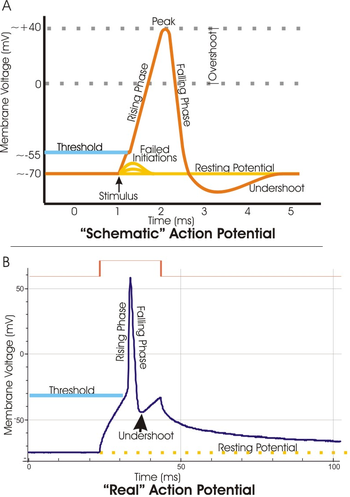
An action potential is a rapid swing in the polarity of the voltage from negative to positive and back, the entire cycle lasting a few milliseconds. Each cycle—and therefore each action potential—has a rising phase, a falling phase, and finally an undershoot (see Action potential phases). In specialized muscle cells of the heart, such as cardiac pacemaker cells, a plateau phase of intermediate voltage may precede the falling phase, extending the action potential duration into hundreds of milliseconds.
Action potentials are measured with the recording techniques of electrophysiology and more recently with neurochips containing EOSFETs. An oscilloscope recording the membrane potential from a single point on an axon shows each stage of the action potential as the wave passes. These phases trace an arc that resembles a distorted sine wave. Its amplitude depends on whether the action potential wave has reached that point on the membrane or has passed it and if so, how long ago.
The action potential does not dwell in one location of the cell's membrane, but travels along the membrane (see Propagation). It can travel along an axon for long distances, for example to carry signals from the spinal cord to the muscles of the foot. In large animals, such as giraffes and whales, the distance traveled can be many meters.
Both the speed and complexity of action potentials vary between different types of cells. However, the amplitudes of the voltage swings tend to be roughly the same. Within any one cell, consecutive action potentials typically are indistinguishable. Neurons are thought to transmit information by generating sequences of action potentials called "spike trains". By varying both the rate as well as the precise timing of the action potentials they generate, neurons can change the information that they transmit.
Underlying mechanism
Resting potential
The potential difference that exists across the membrane of all cells is usually negative inside the cell with respect to the outside. The membrane is said to be polarized. The potential difference across the membrane at rest is called the resting potential and is approximately -70 mV in neurons, with the negative sign indicating that the inside of the cell is negative with respect to the outside. The establishment of this potential difference involves several factors, most importantly the transport of ions across the cell membrane and the selective permeability of the membrane to these ions.
The active transport of potassium and sodium ions into and out of the cell, respectively, is accomplished by a number of sodium-potassium pumps scattered across the cell membrane. Each pump transports two ions of potassium into the cell for every three ions of sodium pumped out. This establishes a particular distribution of positively charged ions across the cell membrane, with more sodium present outside the cell than inside, and more potassium inside the cell than outside. In some situations, the electrogenic sodium-potassium pumps make a significant contribution to the resting membrane potential, but in most cells there are potassium leakage channels that dominate the value of the resting potential.
Sodium and potassium ions diffuse through open ion channels under the influence of their electrochemical gradients. At the resting potential, the net movement of sodium into the cell equals the net movement of potassium out of the cell. However, the resting cell membrane is approximately 75 times more permeable to potassium than to sodium, due to potassium leak channels that are always open. As a result, the cell's resting membrane potential is closer to the equilibrium potential of potassium (=EK=−90 mV) than the equilibrium potential of sodium (=ENa=+45 mV). The cell's resting potential is roughly -70 mV.
Like the resting potential, action potentials of many neurons depend upon the permeability of the cell membrane to sodium and potassium ions.
Phases
The sequence of events that underlie the action potential are outlined below:
Resting potential
At resting potential some potassium leak channels are open but the voltage-gated sodium channels are closed. Potassium diffusing down the potassium concentration gradient creates a negative-inside membrane potential.
Stimulation
A local membrane depolarization caused by an excitatory stimulus causes some voltage-gated sodium channels in the neuron cell surface membrane to open and therefore sodium ions diffuse in through the channels along their electrochemical gradient. Being positively charged, they begin a reversal in the potential difference across the membrane from negative-inside to positive-inside. Initially, the inward movement of sodium ions is also favored by the negative-inside membrane potential.
Rising phase
As sodium ions enter and the membrane potential becomes less negative, more sodium channels open, causing an even greater influx of sodium ions. This is an example of positive feedback. As more sodium channels open, the sodium current dominates over the potassium leak current and the membrane potential becomes positive inside.
Peak
Establishment of a membrane potential of around +30 mV closes the voltage-sensitive inactivation gates of the sodium channels, which are sensitive to the now-positive membrane potential gradient, preventing further influx of sodium. While this occurs, the voltage-sensitive activation gates on the voltage-gated potassium channels begin to open.
Falling phase
As voltage-gated potassium channels open, there is a large outward movement of potassium ions driven by the potassium concentration gradient and initially favored by the positive-inside electrical gradient. As potassium ions diffuse out, this movement of positive charge causes a reversal of the membrane potential to negative-inside and repolarization of the neuron back towards the large negative-inside resting potential.
Undershoot
Closing of voltage-gated potassium channels is both voltage- and time-dependent. As potassium exits the cell, the resulting membrane repolarization initiates the closing of voltage-gated potassium channels. These channels do not close immediately in response to a change in membrane potential. Rather, voltage-gated potassium channels (also called delayed rectifier potassium channels) is delayed. As a result, potassium continues to flow out of the cell even after the membrane has fully repolarized. Thus the membrane potential dips below the normal resting membrane potential of the cell for a brief moment; this dip of hyperpolarization is known as the undershoot.
Threshold and initiation
Action potentials are triggered when an initial depolarization reaches threshold. This threshold potential varies, but generally is about 15 millivolts above the cell's resting membrane potential, occurring when the inward sodium current exceeds the outward potassium current. The net influx of positive charges carried by sodium ions depolarizes the membrane potential, leading to the further opening of voltage-gated sodium channels. These channels support greater inward current causing further depolarization, creating a positive-feedback cycle that drives the membrane potential to a very depolarized level.
The action potential threshold can be shifted by changing the balance between sodium and potassium currents. For example, if some of the sodium channels are in an inactivated state, then a given level of depolarization will open fewer sodium channels and a greater depolarization will be needed to trigger an action potential. This is the basis for the refractory period (see Refractory period).
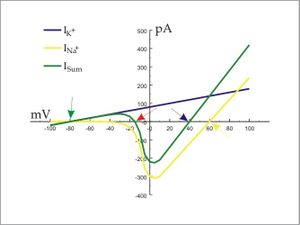
Action potentials are largely dictated by the interplay between sodium and potassium ions (although there are minor contributions from other ions such as calcium and chloride), and are often modeled using hypothetical cells containing only two transmembrane ion channels (a voltage-gated sodium channel and a non-voltage-gated potassium channel). The origin of the action potential threshold may be studied using I/V curves (right) that plot currents through ion channels against the cell's membrane potential. (Note that the illustrated I/V is an "instantaneous" current voltage relationship. It represents the peak current through channels at a given voltage before any inactivation has taken place (i.e. ~ 1 ms after stepping to that voltage for the Na current. The most positive voltages in this plot are only attainable by the cell through artificial means - i.e. voltages imposed by the voltage-clamp apparatus).
Four significant points in the I/V curve are indicated by arrows in the figure:
- The green arrow indicates the resting potential of the cell and also the value of the equilibrium potential for potassium (Ek). As the K+ channel is the only one open at these negative voltages, the cell will rest at Ek. Note that a stable resting potential will be present at any voltage where the summed I/V (green line) crosses the zero current (x-axis) point with a positive slope, such as at the green arrow. Consider why: any perturbation of the membrane potential in the negative direction will result in inward current that will depolarize the cell back toward the crossing point, while, any perturbation of the membrane potential in the positive direction will result in an outward current that will hyperpolarize the cell back toward the crossing point. Thus, any perturbation of the membrane potential around a positive slope crossing will tend to return the voltage to that crossing value.
- The yellow arrow indicates the equilibrium potential for Na+ (ENa). In this two-ion system, ENa is the natural limit of membrane potential beyond which a cell cannot pass. Current values illustrated in this graph that exceed ENa are measured by artificially pushing the cell's voltage past its natural limit. Note however, that ENa could only be reached if the potassium current were absent.
- The blue arrow indicates the maximum voltage that the peak of the action potential can approach. This is the actual natural maximum membrane potential that this cell can reach. It cannot reach ENa because of the counteracting influence of the potassium current.
- The red arrow indicates the action potential threshold. This is where Isum becomes net-inward. Note that this is a zero-current crossing, but with a negative slope. Any such "negative slope crossing" of the zero current level in an I/V plot is an unstable point. At any voltage negative to this crossing, the current is outward and so a cell will tend to return to its resting potential. At any voltage positive of this crossing, the current is inward and will tend to depolarize the cell. This depolarization leads to more inward current, thus the sodium current become regenerative. The point at which the green line reaches its most negative value is the point where all sodium channels are open. Depolarizations beyond that point thus decrease the sodium current as the driving force decreases as the membrane potential approaches ENa.
The action potential threshold is often confused with the "threshold" of sodium channel opening. This is incorrect, because sodium channels have no threshold. Instead, they open in response to depolarization in a stochastic manner. Depolarization does not so much open the channel as increases the probability of it being open. Even at hyperpolarized potentials, a sodium channel will open very occasionally. In addition, the threshold of an action potential is not the voltage at which sodium current becomes significant; it is the point where it exceeds the potassium current.
Biologically in neurons, depolarization typically originates in the dendrites at synapses. In principle, however, an action potential may be initiated anywhere along a nerve fibre. In his discovery of "animal electricity," Luigi Galvani made a leg of a dead frog kick as in life by touching a sciatic nerve with his scalpel, to which he had inadvertently transferred a negative, static-electric charge, thus initiating an action potential.
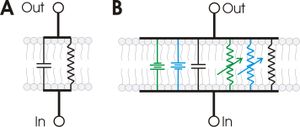
Circuit model
Cell membranes that contain ion channels can be modeled as RC circuits to better understand the propagation of action potentials in biological membranes. In such a circuit, the resistor represents the membrane's ion channels, while the capacitor models the insulating lipid membrane. Variable resistors are used for voltage-gated ion channels, as their resistance changes with voltage. A fixed resistor represents the potassium leak channels that maintain the membrane's resting potential. The sodium and potassium gradients across the membrane are modeled as voltage sources ( batteries).
Propagation
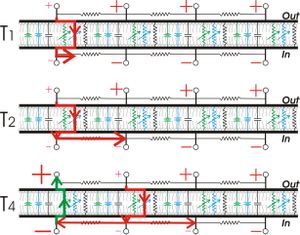
In unmyelinated axons, action potentials propagate as an interaction between passively spreading membrane depolarization and voltage-gated sodium channels. When one patch of cell membrane is depolarized enough to open its voltage-gated sodium channels, sodium ions enter the cell by facilitated diffusion. Once inside, positively-charged sodium ions "nudge" adjacent ions down the axon by electrostatic repulsion (analogous to the principle behind Newton's cradle) and attract negative ions away from the adjacent membrane. As a result, a wave of positivity moves down the axon without any individual ion moving very far. Once the adjacent patch of membrane is depolarized, the voltage-gated sodium channels in that patch open, regenerating the cycle. The process repeats itself down the length of the axon, with an action potential regenerated at each segment of membrane.
Speed of propagation
Action potentials propagate faster in axons of larger diameter, other things being equal. They typically travel from 10-100 m/s. The main reason is that the axial resistance of the axon lumen is lower with larger diameters, because of an increase in the ratio of cross-sectional area to membrane surface area. As the membrane surface area is the chief factor impeding action potential propagation in an unmyelinated axon, increasing this ratio is a particularly effective way of increasing conduction speed.
An extreme example of an animal using axon diameter to speed action potential conduction is found in the Atlantic squid. The squid giant axon controls the muscle contraction associated with the squid's predator escape response. This axon can be more than 1 mm in diameter, and is presumably an adaptation to allow very fast activation of the escape behaviour. The velocity of nerve impulses in these fibers is among the fastest in nature. Squids are notable examples of organisms with unmyelinated axons; the first tests to try to determine the mechanism by which impulses travel along axons, involving the detection of a potential difference between the inside and the surface of a neuron, were undertaken in the 1940s by Alan Hodgkin and Andrew Huxley using squid giant axons because of their relatively large axon diameter. Hodgkin and Huxley won their shares of the 1963 Nobel Prize in Physiology or Medicine for their work on the electrophysiology of nerve action potentials.
In the autonomic nervous system in mammals, postganglionic neurons are unmyelinated. The small diameter of these axons (about 2 µ) results in a propagatory speed of approximately 1 m/s, as opposed to approximately 18 m/s in myelinated nerve fibers of comparable diameter, thus highlighting the effect of myelination on the speed of transmission of impulses.
Saltatory conduction
In myelinated axons, saltatory conduction is the process by which an action potential appears to jump along the length of an axon, being regenerated only at uninsulated segments (the nodes of Ranvier). Saltatory conduction increases nerve conduction velocity without having to dramatically increase axon diameter.
Saltatory conduction has played an important role in the evolution of larger and more complex organisms whose nervous systems must rapidly transmit action potentials across greater distances. Without saltatory conduction, conduction velocity would need large increases in axon diameter, resulting in organisms with nervous systems too large for their bodies.
Detailed mechanism
The main impediment to conduction speed in unmyelinated axons is membrane capacitance. In an electric circuit, the capacity of a capacitor can be decreased by decreasing the cross-sectional area of its plates, or by increasing the distance between plates. The nervous system uses myelin as its main strategy to decrease membrane capacitance. Myelin is an insulating sheath wrapped around axons by Schwann cells and oligodendrocytes, neuroglia that flatten their cytoplasm to form large sheets made up mostly of plasma membrane. These sheets wrap around the axon, moving the conducting plates (the intra- and extracellular fluid) farther apart to decrease membrane capacitance.
The resulting insulation allows the rapid (essentially instantaneous) conduction of ions through a myelinated segment of axon, but prevents the regeneration of action potentials through those segments. Action potentials are only regenerated at the unmyelinated nodes of Ranvier which are spaced intermittently between myelinated segments. An abundance of voltage-gated sodium channels on these bare segments (up to four orders of magnitude greater than their density in unmyelinated axons ) allows action potentials to be efficiently regenerated at the nodes of Ranvier.
As a result of myelination, the insulated portion of the axon behaves like a passive wire: it conducts action potentials rapidly because its membrane capacitance is low, and minimizes the degradation of action potentials because its membrane resistance is high. When this passively propagated signal reaches a node of Ranvier, it initiates an action potential, which subsequently travels passively to the next node where the cycle repeats.
Resilience to injury
The length of myelinated segments of axon is important to saltatory conduction. They should be as long as possible to maximize the length of fast passive conduction, but not so long that the decay of the passive signal is too great to reach threshold at the next node of Ranvier. In reality, myelinated segments are long enough for the passively propagated signal to travel for at least two nodes while retaining enough amplitude to fire an action potential at the second or third node. Thus, the safety factor of saltatory conduction is high, allowing transmission to bypass nodes in case of injury.
Role in disease
Some diseases degrade saltatory conduction and reduce the speed of action potential conductance. The most well-known of these diseases is multiple sclerosis, in which the breakdown of myelin impairs coordinated movement.
Refractory period
Where membrane has undergone an action potential, a refractory period follows. Thus, although the passive transmission of action potentials across myelinated segments would suggest that action potentials propagate in either direction, most action potentials travel unidirectionally because the node behind the propagating action potential is refractory.
This period arises primarily because of the voltage-dependent inactivation of sodium channels, as described by Hodgkin and Huxley in 1952. In addition to the voltage-dependent opening of sodium channels, these channels are also inactivated in a voltage-dependent manner. Immediately after an action potential, during the absolute refractory period, virtually all sodium channels are inactivated and thus it is impossible to fire another action potential in that segment of membrane.
With time, sodium channels are reactivated in a stochastic manner. As they become available, it becomes possible to fire an action potential, albeit one with a much higher threshold. This is the relative refractory period and together with the absolute refractory period, lasts approximately five milliseconds.
Evolutionary purpose
The action potential, as a method of long-distance communication, fits a particular biological need seen most readily when considering the transmission of information along a nerve axon. To move a signal from one end of an axon to the other, nature must contend with physics similar to those that govern the movement of electrical signals along a wire. Due to the resistance and capacitance of a wire, signals tend to degrade as they travel along that wire over a distance. These properties, known collectively as cable properties set the physical limits over which signals can travel. Thus, nonspiking neurons (which carry signals without action potentials) tend to be small. Proper function of the body requires that signals be delivered from one end of an axon to the other without loss. An action potential does not so much propagate along an axon, as it is newly regenerated by the membrane voltage and current at each stretch of membrane along its path. In other words, the nerve membrane recreates the action potential at its full amplitude as it travels down the axon, thus overcoming the limitations imposed by cable physics.
Plant action potentials
Many plants also exhibit action potentials that travel via their phloem to coordinate activity. The main difference between plant and animal action potentials is that plants primarily use potassium and calcium currents while animals typically use currents of potassium and sodium.