Chemical synapse
2007 Schools Wikipedia Selection. Related subjects: General Biology
Chemical synapses are specialized junctions through which cells of the nervous system signal to one another and to non-neuronal cells such as muscles or glands. A chemical synapse between a motor neuron and a muscle cell is called a neuromuscular junction.
Chemical synapses allow the neurons of the central nervous system to form interconnected neural circuits. They are thus crucial to the biological computations that underlie perception and thought. They also provide the means through which the nervous system connects to and controls the other systems of the body.
The human brain contains a huge number of chemical synapses, with young children having about 1016 synapses (10,000 trillion.). This number declines with age, stabilizing by adulthood. Estimates for an adult vary from 1015 to 5 × 1015 synapses (1,000 to 5,000 trillion).
The word "synapse" comes from "synaptein" which Sir Charles Scott Sherrington and his colleagues coined from the Greek "syn-" meaning "together" and "haptein" meaning "to clasp". Chemical synapses are not the only type of biological synapse: electrical and immunological synapses exist as well. Without a qualifier, however, "synapse" by itself most commonly refers to a chemical synapse.
Anatomy
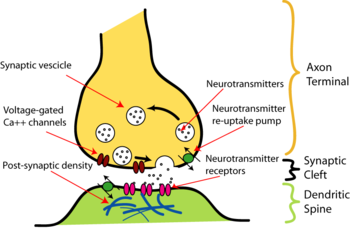
At a prototypical chemical synapse, such as those found at dendritic spines, a mushroom-shaped bud projects from each of two cells and the caps of these buds press flat against one another. At this interface, the membranes of the two cells flank each other across a slender gap, the narrowness of which enables signalling molecules known as neurotransmitters to pass rapidly from one cell to the other by diffusion. This gap, which is about 20 nm wide, is known as the synaptic cleft.
Such synapses are asymmetric both in structure and in how they operate. Only the so-called pre-synaptic neuron secretes the neurotransmitter, which binds to receptors facing into the synapse from the post-synaptic cell. The pre-synaptic nerve terminal (also called the synaptic button or bouton) generally buds from the tip of an axon, while the post-synaptic target surface typically appears on a dendrite, a cell body, or another part of a cell. The parts of synapses where neurotransmitter is released are called the active zones. At active zones the membranes of the two adjacent cells are held in close contact by cell adhesion proteins. Immediately behind the post-synaptic membrane is an elaborate complex of interlinked proteins called the postsynaptic density. Proteins in the postsynaptic density serve a myriad of roles, from anchoring and trafficking neurotransmitter receptors into the plasma membrane, to anchoring various proteins which modulate the activity of the receptors. The postsynaptic cell need not be a neuron, and can also be gland or muscle cells.
Signaling across chemical synapses
The release of neurotransmitter is triggered by the arrival of a nerve impulse (or action potential) and occurs through an unusually rapid process of cellular secretion, also known as exocytosis: Within the pre-synaptic nerve terminal, vesicles containing neurotransmitter sit "docked" and ready at the synaptic membrane. The arriving action potential produces an influx of calcium ions through voltage-dependent, calcium-selective ion channels. Calcium ions then trigger a biochemical cascade which results in vesicles fusing with the presynaptic-membrane and releasing their contents to the synaptic cleft. Vesicle fusion is driven by the action of a set of proteins in the presynaptic terminal known as SNAREs. The membrane added by this fusion is later retrieved by endocytosis and recycled for the formation of fresh neurotransmitter-filled vesicles. Receptors on the opposite side of the synaptic gap bind neurotransmitter molecules and respond by opening nearby ion channels in the post-synaptic cell membrane, causing ions to rush in or out and changing the local transmembrane potential of the cell. The resulting change in voltage is called a postsynaptic potential. In general, the result is excitatory, in the case of depolarizing currents, or inhibitory in the case of hyperpolarizing currents. Whether a synapse is excitatory or inhibitory depends on what type(s) of ion channel conduct the post-synaptic current display(s), which in turn is a function of the type of receptors and neurotransmitter employed at the synapse.
Modulation of synaptic transmission
Following fusion of the synaptic vesicles and release of transmitter molecules into the synaptic cleft, the neurotransmitter is rapidly cleared from the space for recycling by specialized membrane proteins in the pre-synaptic or post-synaptic membrane. This " re-uptake" prevents " desensitization" of the post-synaptic receptors and ensures that succeeding action potentials will elicit the same size post-synaptic potential ("PSP"). The necessity of re-uptake and the phenomenon of desensitization in receptors and ion channels means that the strength of a synapse may in effect diminish as a train of action potentials arrive in rapid succession--a phenomenon that gives rise to the so-called frequency dependence of synapses. The nervous system exploits this property for computational purposes, and can tune its synapses through such means as phosphorylation of the proteins involved. The size, number and replenishment rate of vesicles also are subject to regulation, as are many other elements of synaptic transmission. For example, a class of drugs known as selective serotonin re-uptake inhibitors or SSRIs affect certain synapses by inhibiting the re-uptake of the neurotransmitter serotonin. In contrast, one important excitatory neurotransmitter, acetylcholine, does not undergo re-uptake, but instead is removed from the synapse by the action of the enzyme acetylcholinesterase.
Integration of synaptic inputs
Generally, if an excitatory synapse is strong, an action potential in the pre-synaptic neuron will trigger another in the post-synaptic cell; whereas at a weak synapse the excitatory post-synaptic potential ("EPSP") will not reach the threshold for action potential initiation. In the brain, however, each neuron typically forms synapses with many others, and likewise each receives synaptic inputs from many others. When action potentials fire simultaneously in several neurons that weakly synapse on a single cell, they may initiate an impulse in that cell even though the synapses are weak. This process is known as summation. On the other hand, a pre-synaptic neuron releasing an inhibitory neurotransmitter such as GABA can cause inhibitory postsynaptic potential in the post-synaptic neuron, decreasing its excitability and therefore decreasing the neuron's likelihood to fire an action potential. In this way the output of a neuron may depend on the input of many others, each of which may have a different degree of influence, depending on the strength of its synapse with that neuron. John Carew Eccles performed some of the important early experiments on synaptic integration, for which he received the Nobel Prize for Physiology or Medicine in 1963. Complex input/output relationships form the basis of transistor-based computations in computers, and are thought to figure similarly in neural circuits.
Synaptic strength
The strength of a synapse is defined by the change in transmembrane potential resulting from activation of the postsynaptic neurotransmitter receptors. This change in voltage is known as a post-synaptic potential, and is a direct result of ionic currents flowing through the post-synaptic receptor-channels. Changes in synaptic strength can be short-term and without permanent structural changes in the neurons themselves, lasting seconds to minutes - or long-term ( long-term potentiation, or LTP), in which repeated or continuous synaptic activation can result in second messenger molecules initiating protein synthesis in the neuron's nucleus, resulting in alteration of the structure of the synapse itself. Learning and memory are believed to result from long-term changes in synaptic strength, via a mechanism known as synaptic plasticity.
Relationship to electrical synapses
An electrical synapse is a mechanical and electrically conductive link between two abutting neurons that is formed at a narrow gap between the pre- and postsynaptic cells known as a gap junction. At gap junctions, cells approach within about 3.5 nm of each other (Kandel et al., 2000, p. 179), a much shorter distance than the 20 to 40 nm distance that separates cells at chemical synapses (Hormuzdi et al., 2004). As opposed to chemical synapses, the postsynaptic potential in electrical synapses is not caused by the opening of ion channels by chemical transmitters, but by direct electrical coupling between both neurons. Electrical synapses are therefore faster and more reliable than chemical synapses. Electrical synapses are found throughout the nervous system, yet are less common than chemical synapses.