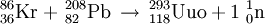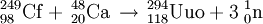Ununoctium
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
||||||
| General | ||||||
|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununoctium, Uuo, 118 | |||||
| Chemical series | noble gases | |||||
| Group, Period, Block | 18, 7, p | |||||
| Appearance | unknown, probably colorless | |||||
| Atomic mass | predicted, (314) g/mol | |||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 7p6 (guess based on radon) |
|||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 8 | |||||
| Phase | presumably a gas | |||||
| CAS registry number | 54144-19-3 | |||||
| References | ||||||
Ununoctium ( IPA pronunciation: /ˌjuːnəˈnɒktiəm/ ) is the temporary IUPAC name for the superheavy element having atomic number of 118, currently the highest atomic number assigned to a reputedly discovered element (see elements discovered in the 21st Century). It has the temporary IUPAC element symbol Uuo.
Ununoctium probably shares similar properties of its group, the noble gases, resembling radon in its chemical properties, and so some researchers have referred to it as eka-radon. It is probably the second radioactive gaseous element and the first standard semiconductive gas.
Ignoring nuclear instability due to radioactivity, scientists expect that ununoctium is much more chemically reactive than xenon or radon. It would likely form stable oxides (UuoO3, etc.) as well as chlorides and fluorides.
History
The name ununoctium is used as a placeholder, as in scientific articles about the search for element 118. Transuranic elements (those beyond uranium) are, except for microscopic quantities, always artificially produced, and usually end up being named for a scientist or the location of a laboratory that does work in atomic physics (see systematic element name for more information). In 1999, researchers at Lawrence Berkeley National Laboratory announced the discovery of elements 116 and 118, in a paper published in Physical Review Letters.
The researchers claimed to have performed the reaction:
The following year, they published a retraction after other researchers were unable to duplicate the results. In June 2002, the director of the lab announced that the original claim of the discovery of these two elements had been based on data fabricated by principal author Victor Ninov.
The American group had intended to name it ghiorsium after Albert Ghiorso before having to retract their claim.
On October 10, 2006, researchers working at the Joint Institute for Nuclear Research ( JINR) in Dubna, Russia, announced in Physical Review C that they had indirectly detected ununoctium-294 produced via collisions of californium-249 atoms and calcium-48 ions :
The research team consisted of workers from JINR and the Lawrence Livermore National Laboratory in California, USA. The decay products of three atoms of ununoctium, not the atoms themselves, were observed in Dubna. A half-life of 0.89 ms was observed: 294Uuo decays into 290Uuh by alpha decay. 290Uuh is very unstable, decaying within a fraction of a second into 286Uuq, which may undergo spontaneous fission or undergo alpha decay into 282Uub, which will undergo spontaneous fission.