Polonium
2007 Schools Wikipedia Selection. Related subjects: Chemical elements
|
|||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
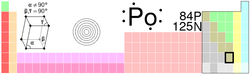
| Name, Symbol, Number | polonium, Po, 84 | ||||||||||||||||||||||||||||||
| Chemical series | metalloids | ||||||||||||||||||||||||||||||
| Group, Period, Block | 16, 6, p | ||||||||||||||||||||||||||||||
| Appearance | silvery | ||||||||||||||||||||||||||||||
| Atomic mass | (209) g/mol | ||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p4 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 6 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||
| Density (near r.t.) | (alpha) 9.196 g·cm−3 | ||||||||||||||||||||||||||||||
| Density (near r.t.) | (beta) 9.398 g·cm−3 | ||||||||||||||||||||||||||||||
| Melting point | 527 K (254 ° C, 489 ° F) |
||||||||||||||||||||||||||||||
| Boiling point | 1235 K (962 ° C, 1764 ° F) |
||||||||||||||||||||||||||||||
| Heat of fusion | ca. 13 kJ·mol−1 | ||||||||||||||||||||||||||||||
| Heat of vaporization | 102.91 kJ·mol−1 | ||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 26.4 J·mol−1·K−1 | ||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||
| Crystal structure | cubic | ||||||||||||||||||||||||||||||
| Oxidation states | 4, 2 ( amphoteric oxide) |
||||||||||||||||||||||||||||||
| Electronegativity | 2.0 (Pauling scale) | ||||||||||||||||||||||||||||||
| Ionization energies | 1st: 812.1 kJ/mol | ||||||||||||||||||||||||||||||
| Atomic radius | 190 pm | ||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 135 pm | ||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||
| Magnetic ordering | nonmagnetic | ||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) (α) 0.40 µΩ·m | ||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) ? 20 W·m−1·K−1 | ||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 23.5 µm·m−1·K−1 | ||||||||||||||||||||||||||||||
| CAS registry number | 7440-08-6 | ||||||||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||
Polonium ( IPA: /pə(ʊ)ˈləʊniəm/) is a chemical element in the periodic table that has the symbol Po and atomic number 84. A rare radioactive metalloid, polonium is chemically similar to tellurium and bismuth and occurs in uranium ores. Polonium has been studied for possible use in heating spacecraft. It exists as a number of isotopes.
Applications
When it is mixed or alloyed with beryllium, polonium can be a neutron source. Beryllium releases a neutron upon absorption of an alpha particle that is supplied by polonium-210. It has been used in this capacity as a neutron trigger for nuclear weapons. Other uses include:
- Devices that eliminate static charges in textile mills and other places. However, beta sources are more commonly used and are less dangerous.
- Brushes that remove accumulated dust from photographic films. The polonium used in these brushes is sealed and controlled thus minimizing radiation hazards.
- As 210Po, a lightweight heat source to power thermoelectric cells.
History
Also called "Radium F", polonium was discovered by Marie Curie and her husband Pierre Curie in 1897 and was later named after Marie's homeland of Poland (Latin: Polonia). Poland at the time was under Russian, Prussian and Austrian domination, and not recognized as an independent country. It was Marie's hope that naming the element after her home land would add notoriety to its plight. Polonium may be the first element named to highlight a political controversy.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity. The pitchblende, after removal of uranium and radium, was more radioactive than both radium and uranium put together. This spurred them on to find the element. The electroscope showed it separating with bismuth.
Occurrence
A very rare element in nature, polonium is found in uranium ores at about 100 micrograms per metric ton (1:1010). Its natural abundance is approximately 0.2% of the abundance of radium.
In 1934 an experiment showed that when natural 209Bi is bombarded with neutrons, 210Bi, which is the parent of polonium, was created. Polonium may now be made in milligram amounts in this procedure which uses high neutron fluxes found in nuclear reactors. Only about 100 grams is believed to be produced each year, making polonium exceedingly rare.
Polonium has been found in tobacco smoke from tobacco leaves grown with phosphate fertilizers.
Isotopes
Polonium has 25 known isotopes all of which are radioactive. They have atomic masses that range from 194 u to 218 u. 210Po is the most widely available. 209Po ( half-life 103 years) and 208Po (half-life 2.9 years) can be made through the alpha, proton, or deuteron bombardment of lead or bismuth in a cyclotron. However these isotopes are expensive to produce.
All elements containing 84 or more protons are radioactive. Alpha decay is a common form of decay for these nuclei. The most stable isotopes with more than 84 protons are 232Th and 238U; which form an " island of stability" which renders them stable enough to be found in large quantities in nature, but heavier nuclei are more and more affected by spontaneous fission.
Polonium-210
This isotope of polonium is an alpha emitter that has a half-life of 138.376 days. A milligram of 210Po emits as many alpha particles as 5 grams of radium. A great deal of energy is released by its decay with half a gram quickly reaching a temperature above 750 K. A few curies ( gigabecquerels) of 210Po emit a blue glow which is caused by excitation of surrounding air. A single gram of 210Po generates 140 watts of power. Because it emits many alpha particles, which are stopped within a very short distance in dense media and release their energy, 210Po has been used as a lightweight heat source to power thermoelectric cells in artificial satellites. A 210Po heat source was also used in each of the Lunokhod rovers deployed on the surface of the Moon, to keep their internal components warm during the lunar nights. Some anti-static brushes contain up to 500 microcuries of 210Po as a source of charged particles for neutralizing static electricity in materials like photographic film.. Polonium-210 has very rare properties as an unstable isotope, as it decays only by emission of an alpha particle, not by emission of an alpha particle and a gamma ray.
Chemical characteristics
Polonium dissolves readily in dilute acids, but is only slightly soluble in alkalis. It is closely related chemically to bismuth and tellurium. Polonium-210 (in common with 238Pu) has the ability to become airborne with ease ( volatilize), 50% of a sample is vaporized in air in 45 hours at 328K (55°C, 131°F) even though its melting point is 527K (254°C, 489°F) and its boiling point is 1235K (962°C, 1763°F). More than one hypothesis exists for how polonium does this; one suggestion is that small clusters of polonium atoms are spalled off by the alpha decay.
It has been reported that microbes can methylate polonium by the action of methylcobalamin.
Solid state form
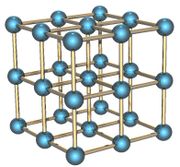
The alpha form of solid polonium is cubic with a distance of 3.352 Å between atoms. It is a simple cubic solid which is not interpenetrated.
The beta form of polonium is hexagonal; it has been reported in the chemical literature, along with the alpha form, several times.
Two papers report X-ray diffraction experiments on polonium metal. The first report of the crystal structure of polonium was done using electron diffraction.
Tests
By means of radiometric methods such as gamma spectroscopy (or a method using a chemical separation followed by an activity measurement with a non-energy-dispersive counter), it is possible to measure the concentrations of radioisotopes and to distinguish one from another. In practice, background noise would be present and depending on the detector, the line width would be larger which would make it harder to identify and measure the isotope. In biological/medical work it is common to use the natural 40K present in all tissues/body fluids as a check of the equipment and as an internal standard.
Toxicity
Polonium is a highly radioactive and toxic element and is very difficult to handle. Even in milligram or microgram amounts, handling polonium-210 is extremely dangerous, requiring specialized equipment and strict handling procedures. Alpha particles emitted by polonium will damage organic tissue easily if polonium is ingested, inhaled, or absorbed (though they do not penetrate the epidermis and hence are not hazardous if the polonium is outside the body).
To produce a potentially lethal radiation dose of 10 sieverts, if ingested, requires just 0.12 micrograms (millionths of a gram) of polonium-210 (about 525 microcuries of radioactivity). A cube of pure polonium-210 about the size of a written period (0.35 mm wide, or 400 micrograms) would still be 3400 times the lethal dose. These calculations are based on a committed effective dose equivalent (CEDE) of 5.14×10−7 sieverts per becquerel (1.9×103 mrem/microcurie) for ingested 210Po and a specific activity of 1.66×1014 Bq/gram (4.49×103 curies/gram). If the polonium is inhaled, the CEDE is even higher, 2.54×10-6 Sv/Bq or 9.43×103 mrem/microcurie, making the lethal dose just 106 microcuries or 0.026 micrograms.
The maximum allowable body burden for ingested polonium is only 1,100 becquerels (0.03 microcurie), which is equivalent to a particle weighing only 6.8 × 10-12 gram. Weight for weight, polonium is approximately 2.5 × 1011 (250 billion) times as toxic as hydrogen cyanide. The maximum permissible concentration for airborne soluble polonium compounds is about 7,500 Bq/m3 (2 × 10-11 µCi/cm3). The biological halflife of polonium in humans is 30 to 50 days.
Use as a poison
Polonium-210 was allegedly used to murder Alexander Litvinenko, a former Russian FSB counterintelligence officer, administered around November 1, 2006 with death occuring November 23, 2006. According to Pat Troop, chief executive of Britain's Health Protection Agency, traces of polonium were found in Litvenenko's urine, as well as in several locations he had visited shortly before becoming ill.