Lyme disease
2007 Schools Wikipedia Selection. Related subjects: Health and medicine
| Nymphal and adult deer ticks can be carriers of Lyme disease. Nymphs are about the size of a poppy seed. | |
| ICD- 10 | A 69.2 |
| ICD- 9 | 088.81 |
| DiseasesDB | 1531 |
| MedlinePlus | 001319 |
| eMedicine | med/1346 |
Lyme disease or Lyme borreliosis is the most common tick-borne disease in North America and Europe, and the second fastest-growing infectious disease in the United States after AIDS. It is named after the town of Old Lyme, Connecticut where a cluster of cases was identified in 1975, although clinical features of the disease had been described in Europe as early as 1909. Lyme disease has now been reported in 49 of 50 states in the U.S, and on every continent except Antarctica. The cause of Lyme disease is a bacterial infection with a spirochete from the species complex Borrelia burgdorferi sensu lato, which is most often acquired from the bite of an infected Ixodes tick. Borrelia burgdorferi was first identified in 1982 by Willy Burgdorfer, a tick-borne disease expert at Rocky Mountain Labs in Hamilton, Montana. While Borrelia burgdorferi sensu stricto is the predominant cause in the U.S., Lyme disease in Europe is more often caused by Borrelia afzelii or Borrelia garinii.
The disease varies widely in its presentation, which may include a rash and flu-like symptoms in its initial stage, followed by musculoskeletal, arthritic, neurologic, psychiatric and/or cardiac manifestations. Early detection and prompt antibiotic treatment most often result in an excellent prognosis. However early detection is difficult when the characteristic rash is not present, and even those who are diagnosed and treated early may remain symptomatic.
Delayed or inadequate treatment may often lead to a chronic illness that is disabling and difficult to treat. Amid great controversy over diagnosis, testing and treatment, two different standards of care for Lyme disease have emerged.
Symptoms
Lyme disease has many signs and symptoms, but skin signs, arthritis and/or various neurological symptoms are often present. Like syphilis, the symptoms frequently seem to resolve, yet the disease progresses. Conventional therapy is with antibiotics. People who suspect they have been exposed to Lyme disease should consult a doctor with knowledge of the disease immediately.
Acute (early) symptoms that may occur
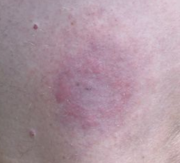
Erythema migrans rash (EM) - Contrary to popular belief, the characteristic "bull's-eye" rash with central clearing is not the most common form. Rashes that are homogeneously red are seen more frequently. Multiple painless EM rashes may occur, indicating disseminated infection. The true incidence of the rash is disputed, with estimates ranging from less than 50% to over 80% of those infected. The symptoms of Lyme disease are fever, malaise, fatigue, headache, muscle and joint aches in large joints, sore throat, sinus infection
Other consequences, include facial paralysis - usually associated with Lyme meningitis or Rocky Mountain spotted fever, palpitations, kidney and intestinal pains.
The incubation period from infection to the onset of symptoms is usually 1–2 weeks, but can be much shorter (a couple of days), or even as long as one month.
Chronic (late) symptoms
- fatigue
- muscle pain (myalgia)
- joint pain with or without frank arthritis
- neuropathy (numbness, tingling, burning, itching, oversensitivity)
- tremor, muscle twitching
- Bell's palsy
- meningitis
- vision problems (eg. double vision)
- sensitivity to light, motion
- hyperacusis (severe sensitivity to sound & vibration)
- vestibular symptoms (balance; inner/middle ear)
- seizures
- severe startle reaction
- panic attacks
- depression
- short-term memory loss
- sleep disturbance
- hallucinations
- cardiac arrhythmias
- tachycardia (too-rapid heartbeat)
- nausea or vomiting
- adrenal disorders
- immune suppression
- acrodermatitis chronica atrophicans (ACA)
The late symptoms of Lyme disease can appear months after infection.
Lyme disease may be misdiagnosed as multiple sclerosis, rheumatoid arthritis, fibromyalgia, chronic fatigue syndrome (CFS), or other (mainly autoimmune and neurological) diseases, which leaves the infection untreated and allows it to further penetrate the organism. Some of these conditions may be misdiagnosed as Lyme disease, although this is thought to be a rare occurrence. False positive Lyme diagnosis is most commonly due to false positive serology in a subset of patients who may suffer from syphillis, rheumatologic diseases, or infectious mononucleosis. More confounding is that patients may present with Lyme Disease and a related disease such as MS. This makes diagnosis exceptionally difficult. It should be noted that this kind of misdiagnosis is the exception rather than the rule as it is widely held that Lyme Disease is underdiagnosed and underreported ranging from factors of 10 to upwards of 40. It is important to remember that chronic fatigue syndrome (CFS) is by definition a diagnosis of exclusion, meaning it would be inaccurate to say that a patient does not have Lyme because he or she has CFS. The substantial overlap in symptomatology between Lyme and CFS makes this a crucial point.
Transmission
Transmission by ticks
Hard-bodied (Ixodes) ticks are the primary Lyme disease vectors. In Europe, Ixodes ricinus, known commonly as the sheep tick, castor bean tick, or European castor bean tick is the transmitter. In North America, Ixodes scapularis ( black-legged tick or deer tick) has been identified as the key to the disease's spread on the east coast, while on the west coast the primary vector is Ixodes pacificus (Western black-legged tick). Another possible vector is Amblyomma americanum (Lone Star tick), which is found throughout the southeastern U.S. as far west as Texas, and increasingly in northeastern states as well.
The longer the duration of tick attachment, the greater the risk of disease transmission, but, typically, for the spirochete to be transferred, the tick must be attached for a minimum of 12 hours, although, only the first part of this statement can be said to be strictly correct. (see Proper Removal of Ticks). Unfortunately only 20% of persons infected with Lyme by the deer tick are aware of any tick bite, making early detection difficult in the absence of a rash. Tick bites usually go unnoticed due to the small size of the tick in its nymphal stage, as well as tick secretions that prevent the host from feeling any itch or pain from the bite. New research suggests that transmission can occur within a few hours of tick attachment, and that the rate of transmission by infected ticks may be much higher than previously assumed.
Congenital Lyme disease
Lyme disease can be transmitted from an infected mother to fetus through the placenta during pregnancy, possibly resulting in stillbirth. The risk of transmission is minimized if the mother receives prompt antibiotic treatment, though physicians disagree as to the duration of treatment required.
Other modes of transmission
There is at least one case report of transmission by a biting fly. Lyme spirochetes have been found in biting flies as well as mosquitos. Some researchers believe biting insects do not feed long enough to transmit the infection, while others including Borrelia burgdorferi discoverer Willy Burgdorfer believe more research is needed. There is also some anecdotal, largely unconfirmed evidence of sexual transmission. Lyme spirochetes have been found in semen and breast milk, though transmission by these routes has yet to be proven.
Microbiology
Strains
Lyme disease is caused by spirochetal bacteria from the genus Borrelia, which has well over three hundred known genomic strains. The Borrelia species known to cause Lyme disease are collectively known as Borrelia burgdorferi sensu lato, and have been found to have greater strain diversity than previously estimated. Until recently it was thought that only three genospecies caused Lyme disease: B. burgdorferi sensu stricto (predominant in North America, but also in Europe), B. afzelii, and B. garinii (both predominant in Eurasia). However, newly discovered genospecies have also been found to cause disease in humans: B. lusitaniae in Europe (especially Portugal), North Africa and Asia, B. bissettii in the U.S. and Europe, and B. spielmanii in Europe. Additional B. burgdorferi sensu lato genospecies suspected of causing illness, but not confirmed by culture, include B. valaisiana ( Eurasia, especially England, Switzerland and the Netherlands); B. japonica, B. tanukii and B. turdae (Japan); B. sinica (China); and B. andersonii ( U.S.). Some of these species are carried by ticks not currently recognized as carriers of Lyme disease. Note: At present, diagnostic tests are based only on B. burgdorferi sensu stricto (the only species used in the U.S.), B. afzelii and B. garinii.
Apart from this group of closely related genospecies, additional Borrelia species of interest include B. lonestari, a spirochete recently detected in the Amblyomma americanum tick (Lone Star tick) in the U.S. B. lonestari is suspected of causing STARI (Southern Tick-Associated Rash Illness), also known as Masters disease in honour of its discoverer. The illness follows a Lone Star tick bite and clinically resembles Lyme disease, but sufferers usually test negative for Lyme. There is currently no diagnostic test available for STARI/Masters, and no official treatment protocol, though antibiotics are generally prescribed. The B. miyamotoi spirochete, related to the relapsing fever group of spirochetes, is also suspected of causing illness in Japan. Spirochetes similar to B. miyamotoi have recently been found in both I. ricinus ticks in Sweden and I. scapularis ticks in the U.S.
Genomic characteristics
One of the most striking features of B. burgdorferi as compared with other eubacteria is its unusual genome, which is far more complex than that of its spirochetal cousin Treponema pallidum, the agent of syphilis. The genome of B. burgdorferi includes a linear chromosome approximately one megabase in size, with 21 plasmids (12 linear and 9 circular) - by far the largest number of plasmids found in any known bacterium. Genetic exchange, including plasmid transfers, contributes to the pathogenicity of the organism. Long-term culture of B. burgdorferi results in a loss of some plasmids and changes in expressed protein profiles. Associated with the loss of plasmids is a loss in the ability of the organism to infect laboratory animals, suggesting that the plasmids encode key genes involved in virulence.
Structure and growth
B. burgdorferi is a highly specialized, motile, two-membrane, spiral-shaped spirochete ranging from about 9 to 32 micrometers in length. It is often described as gram-negative and has an outer membrane with LPS, though it stains only weakly in the Gram stain. B. burgdorferi is a microaerophilic organism, requiring little oxygen to survive. It lives primarily as an extracellular pathogen, although it can also hide intracellularly (see Mechanisms of persistence section).
Like other spirochetes such as T. pallidum (the agent of syphilis), B. burgdorferi has an axial filament composed of flagella which run lengthways between its cell wall and outer membrane. This structure allows the spirochete to move efficiently in corkscrew fashion through viscous media, such as connective tissue. As a result, B. burgdorferi can disseminate throughout the body within days to weeks of infection, penetrating deeply into tissue where the immune system and antibiotics may not be able to eradicate the infection.
B. burgdorferi is very slow growing, with a doubling time of 12-24 hours (in contrast to pathogens such as Streptococcus and Staphylococcus, which have a doubling time of 20-30 minutes). Since most antibiotics kill bacteria only when they are dividing, this longer doubling time necessitates the use of relatively longer treatment courses for Lyme disease. Antibiotics are most effective during the growth phase, which for B. burgdorferi occurs in four-week cycles. Some clinicians have observed that chronic Lyme patients commonly experience a worsening of symptoms every four weeks; these periodic flare-ups are thought to correspond to the growth phase of B. burgdorferi.
Mechanisms of persistence
While B. burgdorferi is susceptible to a number of antibiotics in vitro, there are contradictory reports as to the efficacy of antibiotics in vivo. B. burgdorferi may persist in humans and animals for months or years despite a robust immune response and standard antibiotic treatment, particularly when treatment is delayed and dissemination widespread. Numerous studies have demonstrated persistence of infection despite antibiotic therapy.
Various survival strategies of B. burgdorferi have been posited to explain this phenomenon, including the following:
- Physical sequestration of B. burgdorferi in sites that are inaccessible to the immune system and antibiotics, such as the brain and central nervous system. New evidence suggests that B. burgdorferi may use the host's fibrinolytic system to penetrate the blood-brain barrier.
- Intracellular invasion. B. burgdorferi has been shown to invade a variety of cells, including endothelium, fibroblasts, lymphocytes, macrophages, keratinocytes, synovium, and most recently neuronal and glial cells. By 'hiding' inside these cells, B. burgdorferi is able to evade the immune system and is protected to varying degrees against antibiotics, allowing the infection to persist in a chronic state. Paradoxically, many of these scientific studies were performed and published by critics of persistent Borrelia infection.
- Altered morphological forms, i.e. spheroplasts (cysts, granules).
- The existence of B. burgdorferi spheroplasts, which lack a cell wall, has been well documented in vitro, in vivo, and in an ex vivo model.The fact that energy is required for the spiral bacterium to convert to the cystic form suggests that these altered forms have a survival function, and are not merely end stage degeneration products. The spheroplasts are indeed virulent and infectious, able to survive under adverse environmental conditions, and have been shown to revert back to the spiral form in vitro, once conditions are more favorable.
- A number of other factors make B. burgdorferi spheroplasts a key factor in the relapsing, chronic nature of Lyme disease. Compared to the spiral form, spheroplasts have dramatically reduced surface area for immune surveillance. They also express different surface proteins - another reason for seronegative disease (i.e. false-negative antibody tests), as current tests only look for antibodies to surface proteins of the spiral form. In addition, B. burgdorferi spheroplasts are generally not susceptible to the antibiotics traditionally used for Lyme disease. They have instead shown sensitivity in vitro to antiparasitic drugs such as metronidazole, tinidazole, and hydroxychloroquine, to which the spiral form of B. burgdorferi is not sensitive.
- Antigenic variation. Like the Borrelia that cause relapsing fever, B. burgdorferi has the ability to vary its surface proteins in response to immune attack. This ability is related to the genomic complexity of B. burgdorferi, and is another way B. burgdorferi evades the immune system to establish a chronic infection.
- Immune system suppression. Complement inhibition, induction of anti-inflammatory cytokines such as IL-10, and the formation of immune complexes have all been documented in B. burgdorferi infection. Furthermore, the existence of immune complexes provides another explanation for seronegative disease (i.e. false-negative antibody tests of blood and cerebrospinal fluid), as studies have shown that substantial numbers of seronegative Lyme patients have antibodies bound up in these complexes.
Diagnosis
The most reliable method of diagnosing Lyme disease is a clinical exam by an experienced practitioner, taking into account symptoms, history, and possible exposure to ticks in an endemic area. Clinicians who diagnose strictly based on the U.S. Centers for Disease Control (CDC) Case Definition for Lyme are in error, as the CDC explicitly states that this definition is intended for surveillance purposes only, and is "not intended to be used in clinical diagnosis."
The EM rash, which does not occur in all cases, is considered sufficient to make a diagnosis of Lyme disease and prompt treatment without further testing. In fact because of the undisputed high rate of false negatives during the early stage of the disease (before a sufficient antibody response has been established), it is recommended that tests not be performed when a patient has an EM rash.
The serological laboratory tests available are the Western blot and ELISA. In the two-tiered protocol recommended by the CDC according to their case definition, the ELISA is performed first, and if it is positive or equivocal, a Western blot is then performed to support the diagnosis. The reliability of testing in diagnosis remains controversial (see The Lyme controversy--Testing).
False-positive results for the Western blot IgM are described with varicella-zoster virus, Epstein-Barr virus, cytomegalovirus. and herpes simplex type virus 2. However studies show the Western blot IgM has a specificity of 94-96% for patients with symptoms suggestive of Lyme disease.
False-negative test results have been widely reported in both early and late disease.
Polymerase chain reaction (PCR) tests for Lyme disease may also be available to the patient. A PCR test attempts to detect the genetic material (DNA) of the Lyme disease spirochete, whereas the Western blot and ELISA tests look for antibodies to the organism. PCR tests are rarely susceptible to false-positive results but can often show false-negative results.
Given the testing difficulties described above, some patients are employing a vitamin D metabolites test as an alternative indicator. A finding of a low 25-hydroxyvitamin D level coupled with a high 1,25-dihydroxyvitamin D level can be associated with an infection by B. burgdorferi or other spirochetal bacteria. Since such abnormal vitamin D levels can also be caused by other disease processes, further evaluation is warranted to rule those out before initiating treatment.
Prognosis
For early cases, prompt treatment is usually, but not always, curative. However, the severity and treatment of Lyme disease may be complicated due to late diagnosis, failure of antibiotic treatment, simultaneous infection with other tick-borne diseases including ehrlichiosis, babesiosis, and bartonella, and immune suppression in the patient (sometimes resulting from inappropriate treatment with steroids).
A meta-analysis published in 2005 found that some patients with Lyme disease have fatigue, joint and/or muscle pain, and neurocognitive symptoms persisting for years despite antibiotic treatment. Patients with chronic Lyme disease have been shown to experience a level of physical disability equivalent to that seen in congestive heart failure. The disease is rarely fatal in and of itself, although deaths have been reported.
Treatment
Persons who remove attached ticks should be monitored closely for signs and symptoms of tick-borne diseases for up to 30 days. Single-dose doxycycline therapy may be considered for deer tick bites when the tick has been on the person for at least 36 hours.
Traditional treatment of acute Lyme disease usually consists of a minimum two-week to one-month course of antibiotics. In later stages, the bacteria disseminate throughout the body and may cross the blood-brain barrier, making the infection more difficult to treat. Chronic or late diagnosed Lyme is treated with oral or IV antibiotics, frequently ceftriaxone, for a minimum of four weeks.
With little research conducted specifically on chronic Lyme disease, treatment remains controversial. Currently there are two sets of peer-reviewed published guidelines; the International Lyme and Associated Diseases Society (ILADS) advocates extended courses of antibiotics for chronic Lyme patients, while the Infectious Diseases Society of America does not recognize chronic infection and recommends no treatment for persistent symptoms following infection (see The Lyme controversy--Two standards of care). Double-blind, placebo-controlled trials of long-term antibiotics for chronic Lyme have produced mixed results (see The Lyme controversy--Long-term antibiotic therapy).
A number of alternative therapies have been suggested, though clinical trials have not been conducted. For example, the use of hyperbaric oxygen therapy (which is used conventionally to treat a number of other conditions), as an adjunct to antibiotics for Lyme has been discussed. Though there is no published data from clinical trials to support its use, preliminary results using a murine model suggest its effectiveness against Borrelia burgdorferi both in vitro and in vivo. Alternative medicine approaches include bee venom because it contains the peptide melittin, which has been shown to exert profound inhibitory effects on lyme bacteria in vitro. The herb andrographis, though not specifically studied for Borrelia species, has been found to have both antimalarial and antibacterial properties against a wide range of organisms in vitro and in vivo, leading some herbalists to recommend it for Lyme. Other alternative practitioners recommend large doses of salt combined with vitamin C, based on the theory that this protocol kills bacteria by enhancing the activity of elastase and possibly by other mechanisms, though the safety and efficacy of this approach remains unproven.
The Lyme controversy
Although there is no doubt that Lyme disease exists, and most clinicians agree on the treatment of early Lyme disease, there is considerable controversy as to the prevalence of the disease, the proper procedure for diagnosis and treatment of later stages, and the likelihood of a chronic, antibiotic-resistant Lyme infection. On one side are those who believe that Lyme disease is relatively rare, easily diagnosed with available blood tests, and easily treated with two to four weeks of antibiotics. On the other side are those who believe that Lyme disease is under-diagnosed, that available blood tests are unreliable, and that extended antibiotic treatment is often necessary.
The majority of public health agencies such as the U.S. Centers for Disease Control maintain the former position. While this narrower position is sometimes described as the "mainstream" view of Lyme disease, published studies involving non-randomized surveys of physicians in endemic areas found physicians evenly split in their views, with the majority recognizing seronegative Lyme disease, and roughly half prescribing extended courses of antibiotics for chronic Lyme disease.
Since October 2006, the Lyme controversy has heated up dramatically beginning with the release of updated diagnosis and treatment guidelines from the Infectious Diseases Society of America (IDSA). The new IDSA recommendations are even more restrictive than before, requiring either an EM rash or positive laboratory tests for diagnosis. Seronegative Lyme disease is no longer acknowledged, except in early Lyme. The authors of the guidelines maintain that chronic Lyme disease does not result from persistent infection, and therefore treatment beyond 2-4 weeks is not recommended by the IDSA, even in late stage cases.
The 2006 IDSA guidelines have come under fire from a variety of corners. The International Lyme and Associated Diseases Society (ILADS), a professional medical society, formally requested retraction of the IDSA guidelines, arguing that the authors ignored all published data that conflicted with their opinions, and refused input from physicians and patients with differing views. The all-volunteer Lyme Disease Association, which is the largest Lyme advocacy group in the U.S., expressed concerns that the guidelines do not allow for physicians' clinical discretion, and that with more cases going undiagnosed and untreated by the stricter guidelines, more patients than ever will develop disabling, late-stage Lyme disease.
In an unprecedented move, Connecticut Attorney General Richard Blumenthal initiated a formal investigation into the development of the IDSA guidelines in November 2006. The Attorney General's office is considering whether the IDSA violated antitrust laws through exclusionary conduct and monopolization in the development of the guidelines. "These guidelines were set by a panel that essentially locked out competing points of view," Blumenthal said. "Presumably, the IDSA is a non-profit making organization, but such organizations can still be used for anti-competitive purposes."
Two standards of care
Because the legal standard of care is defined by the consensus of treating physicians (rather than published guidelines), two standards of care for Lyme disease are now recognized in the U.S., a situation with significant legal implications for both patients and clinicians.
| ILADS (The International Lyme and Associated Diseases Society) ILADS Mission Statement |
IDSA (The Infectious Diseases Society of America) IDSA Mission Statement |
|
| Peer-reviewed treatment guidelines | ILADS Guidelines | IDSA Guidelines |
| Public statements | "A small group of scientists...deny the existence of chronic Lyme disease," wrote ILADS president Raphael Stricker, M.D., referring in part to the IDSA. "Fearing 'over-diagnosis,' they publish guidelines endorsing an insensitive testing program that misses half the patients with the tick-borne illness. Fearing 'over-treatment,' they recommend antibiotic therapy barely adequate for acute infection and wholly inadequate for chronic Lyme disease. Soon they will publish the latest version of an already restrictive set of guidelines that will further pressure the Centers for Disease Control and Prevention and academic institutions to ignore chronic Lyme disease. The guidelines will encourage insurance companies to embrace up-front cost savings inherent in shorter treatment and deny payment for longer treatment, even if the Lyme patient is still sick but showing signs of improvement. Although the Lyme denialists claim support from mainstream medical groups, the reality is that the handful of them have managed to dictate policy to larger health care organizations through a closed process that rejects dissenting views." | The IDSA has attacked ILADS as a "special interest group... which represents a few physicians who advocate unconventional treatments based on testimonials rather than scientifically sound clinical trials." (See Clinical Trials). “Nearly all people – more than 95 percent – who do get sick with Lyme disease and are treated with the recommended course of antibiotics get better and go on with their lives,” said Gary Wormser, M.D., lead author of IDSA’s 2006 guidelines on Lyme disease. |
| EM rash | Present less than 50% of the time. Studies that show otherwise are flawed because they rely on circular logic, as subjects must meet CDC criteria which prioritize the rash over other disease manifestations. Among those who would be excluded from such studies are: 1) seronegative Lyme patients without a rash (even if there is definitive evidence of infection such as a positive PCR), 2) seropositive patients without a rash who present with fever, flu-like symptoms, joint and muscle pain, paresthesias and/or encephalopathy (symptoms not included in the restrictive CDC case definition), and 3) late-stage patients whose diagnosis was delayed because no rash was present. The exclusion of these groups leads to an artificially high estimate of the incidence of EM rash among those infected with Lyme. | "The great majority of Lyme patients" present with an EM rash, according to studies of patients with early Lyme disease diagnosed by CDC criteria. |
| Testing | Not reliable, particularly for late cases; used to support a clinical diagnosis (see Testing section for discussion). | Nearly always reliable after the first few weeks of infection. |
| Chronic Lyme disease | Persistent Lyme infection exists due to various mechanisms of antibiotic resistance, particularly when diagnosis and treatment are delayed, as numerous studies have demonstrated (see Mechanisms of persistence section). Lengthy treatment regimens are sometimes required. | Persistent Lyme infection is not recognized. Some patients report continuing and/or relapsing non-specific symptoms such as generalized pain, joint pain or fatigue following an episode of Lyme disease that has been treated with a standard course of antibiotics. “These patients with symptoms that persist for weeks, months or longer appear to be a heterogeneous group, and they report non-specific symptoms that also are associated with a number of other medical diseases, both infectious and noninfectious,” according to Gary Wormser, M.D., lead author of the IDSA guidelines. Post-treatment symptoms are termed "Post-Lyme disease syndrome" and are often attributed to an unspecified autoimmune process and/or the development of fibromyalgia or chronic fatigue syndrome, psychiatric disorders such as somatization, or simply stress. |
| Long-term antibiotic treatment | ILADS maintains that a 2-4 week course of antibiotics is not always curative, particularly when diagnosis is delayed and disease is at a later, disseminated stage. ILADS recommends long-term antibiotic therapy for these symptomatic patients, while acknowledging the lack of published data supporting either long-term or short-term treatment durations. The medical literature provides a compelling rationale for the use of longer regimens for some patients. While more research is needed, treatment should not be withheld from patients in the meantime. (See Evidence section for list of published clinical trials.) | According to the IDSA, virtually all patients are cured of infection with a single course of 14-28 days of antibiotics, regardless of the stage of their illness. Rarely, a second course of treatment is recommended, but long-term antibiotic therapy is not recommended according to IDSA guidelines. Lead author Dr. Gary Wormser cautioned that “there are no convincing published data showing such [long-term] treatment to be effective.” (See Evidence section for list of published clinical trials.) |
| Primary concern regarding misdiagnosis | The under-diagnosis of Lyme may lead to untreated chronic, persistent infection resulting in severe disability and possibly even death (see #Prognosis). | The over-diagnosis of Lyme may lead to the unnecessary use of antibiotics resulting in side effects (most commonly nausea). Where intravenous therapy is used, there are more serious risks including central line infection, which has resulted in the death of one patient being treated for chronic Lyme disease. There are also concerns about the cost of antibiotic treatment. |
| Risk-benefit analysis | The potential harm in letting a persistent Lyme infection go untreated far outweighs the potential side-effects of long-term antibiotic use. If long-term oral antibiotic therapy is considered safe enough for acne patients, its use is certainly justified for chronic Lyme patients. Intravenous therapy is justified for serious, refractory cases or those with clear central nervous system involvement. Risks are minimized by skilled clinicians who take appropriate precautions. | Since chronic Lyme infection is presumed not to exist, any potential adverse effects of long-term antibiotic therapy (both oral and intravenous) outweigh the (non-existent) benefits. According to Gary Wormser, M.D., lead author of the IDSA guidelines, long-term antibiotic therapy may be dangerous and lead to drug-resistant superbugs. |
The CDC case definition
Confusion about the significance of the U.S. Centers for Disease Control Case Definition for Lyme disease lies at the heart of the controversy over diagnosis. The CDC has explicitly stated that the following definition is meant to be used for surveillance purposes, not diagnostic purposes.
- CDC Case Definition for Lyme disease
- Erythema migrans rash (at least 5 cm in diameter)
- - OR -
- Positive blood tests (ELISA followed by Western blot) AND one or more of the following manifestations:
- Recurrent arthritis
- Bell's Palsy or other cranial neuritis, radiculoneuropathy, lymphocytic meningitis, encephalomyelitis, or positive Lyme titer in CSF
- 2nd or 3rd degree heart block
A number of well-documented signs of chronic Lyme disease including encephalopathy (manifested by memory loss, mood changes and sleep disturbance) are not part of the CDC case definition. Therefore clinicians using the CDC criteria for diagnostic purposes will misdiagnose patients who have the disease. Additionally, reliance on the CDC case definition for clinical purposes would result in the misdiagnosis of those with false-negative test results, a widely reported phenomenon (see Diagnosis).
Testing
The debate over Lyme disease testing remains a heated one, with concern over both false-positives and false-negatives (see Diagnosis). Tests currently rely on indirect methods of detection (i.e. the body's immune system response), because it is very difficult to culture the bacteria directly from patients. Specific issues with regard to the testing controversy include the following:
- Sensitivity of the CDC's testing protocol. Critics argue that the CDC's 2-tiered testing protocol ( ELISA test, followed by confirmatory Western blot test if positive or equivocal) misses many patients who are infected. This criticism is not without merit. Several studies have examined this question and found that as many as 50 percent of definite Lyme Disease as defined by the presence of Borrelial DNA or Borrelial culture were negative when tested against the CDC's recommendations. It is important to note that such studies have included both early and late stage Lyme Disease patients. A study from the College of American Pathologists concluded that "these tests will not be useful as screening tests until their sensitivity is improved."
- Inadequate lab standardization. Standardization of testing has been found to be inadequate, with a high degree of interlaboratory variability.
- No diagnostic gold standard to determine sensitivity of tests in late disease. Without a diagnostic gold standard to identify those with chronic Lyme disease, circular reasoning becomes a problem in studies that evaluate the sensitivity of serologic tests for this population. Bias is unavoidable if subjects are selected by CDC criteria, since late-stage patients must have tested positive previously in order to qualify for a study. In a study cited by the CDC to defend the tests' validity, the authors acknowledge this risk of selection bias.
- False negative test results due to the following, particularly in late and chronic Lyme disease:
- Immune system evasion by Borrelia burgdorferi. Intracellular sequestration, antigen variation, immune suppression, the formation of immune complexes, and predominance of cystic forms have all been cited as reasons for seronegativity in late and chronic Lyme disease (see Mechanisms of persistence section).
- Positive test criteria is based on early Lyme disease. The CDC's criteria for a positive Western blot were developed based upon on a study of patients with early Lyme disease. The serologic response of patients with late-stage Lyme disease was not analyzed and incorporated, despite that fact that such cases require a positive Western blot for diagnosis by CDC standards.
- Specific markers for late-stage Lyme disease left out. Several highly specific antibody bands for Lyme (31-kDa and 34-kDa, corresponding to outer surface proteins A and B) were not included in the CDC criteria for a positive Western blot because they only appear late in the disease. It is important to note that these bands which have not been included on the CDC Western Blot are so specific to Borrelia Burgdorferri that they are being used/studied for the development of a Lyme Disease vaccine. As a result, the vast majority of laboratories do not report these bands, even if they are positive. This is one reason some clinicians use laboratories that specialize in tick-borne disease, as they usually report all antibody bands.
- Tests based on only one strain. Current tests at most laboratories are based on only one strain of Borrelia burgdorferi (the B31 strain is used in the U.S.) despite the fact that there are over three hundred strains worldwide and over one hundred in North America (see #Strains). Several studies have found that this practice can lead to false-negatives - another reason some clinicians use tick-borne disease specialty labs, which utilize multiple strains of Borrelia burgdorferi in the preparation of test kits.
- Concern about false-positives. Many physicians with a conservative view of Lyme disease believe it is over-diagnosed and over-treated. One of the most widely cited studies from critics of Lyme Disease was written by Allan Steere. His study, published in JAMA concluded that 57% of patients diagnosed with Chronic Lyme in an endemic area did not actually have the disease. Critics have responded with the following arguments:
- 45% of those considered "misdiagnosed" in the study received positive results from another laboratory, and negative results from the authors' laboratory. However there was no independent evaluation, and no reason to assume that the authors' laboratory was superior. In a separate study funded by the NIH, the laboratory used by Allan Steere was sent definite Lyme Disease serology in a blinded fashion in an attempt to discover the reliability of testing at major academic centers. The study concluded that the rate of true positives for this laboratory was significanly less than 100 percenent.
- The authors failed to consider the phenomenon of seronegative Lyme disease ( false-negatives).
- Rather than consider the possibility of persistent infection, the authors considered treatment failure to be evidence of misdiagnosis, i.e. patients could not possibly have Lyme if they were not cured by a standard course of antibiotics even though the authors had previously published that treatment failures were common. However, despite this fact, the authors concluded that all patients with Lyme respond to treatment - another example of circular reasoning.
- The authors excluded patients from a diagnosis of Lyme disease if they had psychiatric symptoms, despite the fact that Lyme can cause such symptoms.
- Testing positive after treatment. Because the tests measure antibodies to Borrelia burgdorferi and not the organism itself, it is theoretically possible to test positive even if the organism has been eradicated. All agree that no treatment is required in asymptomatic patients regardless of test results; however, controversy arises when a patient continues to have symptoms after a course of treatment. In this scenario, those who hold a conservative view believe the infection must have been eradicated by the treatment, and the positive test no longer indicates active infection but rather a persisting antibody response, regardless of the clinical picture. Those with a broader view of Lyme believe the evidence and clinical picture in this case most likely point to a persisting infection requiring further antibiotic treatment.
Long-term antibiotic therapy
There is little concrete evidence either for or against the use of antibiotics for chronic Lyme disease, because only three such double-blind, placebo-controlled clinical trials have been funded to date by the U.S. National Institutes of Health, with conflicting results.
Evidence from controlled studies
1) Klempner et al. (2001). One month of intravenous ceftriaxone followed by two months of low-dose oral doxycycline or placebo given to chronic Lyme patients with one or more of the following symptoms: musculoskeletal pain, cognitive impairment, radicular pain, paresthesias or dysesthesias.
- No significant benefit found in physical or mental health. However critics maintain that the study contains serious methodological flaws including the following:
- The dose of doxycycline used in the study (200 mg daily) is too low to penetrate the central nervous system; failure was to be expected at this dose.
- This was not in actuality a "long-term" trial as described, but rather a short-term trial of ceftriaxone, because of the sequential use of two antibiotics with different modes of action (and with the second antibiotic inadequately dosed). Since patients had failed similar treatment previously, it was unlikely that this regimen would produce any benefit.
- Cognitive status was measured only subjectively using patient surveys (the SF-36), making it impossible to assess changes in executive functioning often seen in chronic Lyme patients. Objective neuropsychiatric testing results were not reported.
- The authors’ statement that not a single one of 1800 patients screened were PCR positive for Lyme is puzzling in light of numerous studies documenting persisting infection in patients who remain symptomatic after treatment.Either selection bias resulted in a study population that was not representative of chronic Lyme patients (and thus the study is not generalizable), or the accuracy of the authors’ PCR methods is in doubt. In either scenario, the authors' conclusion that chronic Lyme patients do not suffer from persistent infection is invalid.
- The external validity of the study has been questioned on the grounds that the study population was not representative of the general population of chronic Lyme patients - an issue that Klempner et al. did not address in their discussion. The average subject had been ill for 4.7 years and had already failed three courses of treatment. Thus it is argued that the data are not generalizable to all patients with chronic Lyme disease, meaning one can not conclude, as Klempner et al. did, that long-term antibiotic therapy is unhelpful for all chronic Lyme patients.
2) Krupp et al. (2003). Four weeks of intravenous ceftriaxone or placebo given to chronic Lyme patients with "persistent severe fatigue".
- Significant improvement in fatigue. The treatment effect remained even after adjusting for age, pain, history of psychiatric disorder and depressive symptoms.
- No improvement in cognitive symptoms. However the only symptom criteria for entrance into the study was severe fatigue. The authors acknowledge that the patients’ cognitive deficits at baseline were mild, which may explain the lack of treatment effect on cognition.
3) Fallon et al. (not yet published). Results presented on October 22, 2004 at the Columbia University/Lyme Disease Association Conference in Rye, NY . Ten weeks of intravenous ceftriaxone or placebo given to chronic Lyme patients with ongoing memory impairment.
- Significant improvement in both physical and cognitive symptoms. Physical improvement was maintained at 12 weeks followup. Patients relapsed on cognitive measures at followup, suggesting longer regimens may be required.
- Improvements in cognitive functioning correlated with changes in blood flow to the brain as measured by SPECT scans.
It is important to note that Fallon et al's study is the only biological examination of chronic Lyme Disease to date. In the two other studies, results were interpreted using questionnaires, often administered over the phone.
Fallon's study had several blinds. This level of methodology has never before been attempted in a study of chronic Lyme Disease. One of the reasons that many levels of blind were used in Fallon's study has to do with the controversy surrounding Lyme Disease. The aim of this study was to include people for whom there was little disagreement in terms of a correct Lyme Disease diagnosis. Secondly, the strict methodology, though tedious, was required because scientific rigor of a very high degree was necessary given the political nature of Lyme Disease. In this study, patients with chronic Lyme Disease were given SPECT scans before and after treatment. A SPECT scan of the brain qualitatively or quantitatively (depending on the sophistication of the equipment) measures metabolic and blood flow activity within the brain. This is a physical marker that can scientifically examine cause and effect as opposed to questionnaires which are open to the opinions of the participant and influence of the examiner. Patients were also administered purely quantitative examinations aimed at assessing disability, ie: neuropsychological testing. Lastly, as in other studies, patients were asked how they felt after treatment. All of these tests included several degrees of blind, ie: radiologist blind to diagnosis, neuropsychiatrists blind to diagnosis, patient blind to treatment, etc..
Evidence from uncontrolled studies
While the results of placebo-controlled studies are mixed, several uncontrolled studies suggest that longer durations of antibiotic treatment may be beneficial for chronic Lyme disease.
Implications for treatment
The widely publicized results of the Klempner study have led some to proclaim that long-term antibiotics are unhelpful for patients with chronic Lyme disease, warning patients and clinicians that the evidence does not support their use. Others see this as an abuse of the concept of evidence-based medicine. They argue that treatment failure in one questionably designed clinical trial does not justify such warnings in light of other evidence, and that withholding antibiotic treatment is unethical in the face of patient suffering. Since the optimal choice of antibiotic(s) and treatment duration is unknown and may vary by strain, many believe additional research on chronic Lyme disease is needed before strict treatment recommendations can be issued.
Prevention
The best prevention involves avoiding areas in which ticks are found and can reduce the probability of contracting Lyme disease. Other good prevention practices include wearing clothing that covers the entire body when in a wooded area; using mosquito/tick repellent; after exposure to wooded areas, check all parts of the body (including hair) for ticks.
A method of protecting your whole property - Damminix - is also cited. It consists of biodegradable cardboard tubes stuffed with permethrin-treated cotton and works in the following way: Mice collect the cotton for lining their nests. The pesticide on the cotton kills any immature ticks that are feeding on the mice. It is important to put the tubes where mice will find them, such as in dense, dark brush or at the base of a log; mice are unlikely to gather the cotton from an open lawn. Best results are obtained with regular applications early in the spring and again in late summer. The more neighbors who also use Damminix, the better. Damminix appears to help control tick populations, particularly in the year following initial use. Note that it is not effective on the West Coast.
A potential alternative to Damminix, the Maxforce Tick Management system, is based on plastic baitboxes that attract rodents. Rodents entering these baitboxes would then be painted with fipronil. This product requires professional installation. As of June 2006, this product is no longer available. The reason appears to have been that in 2005, there were selective reports of grey squirrels "chewing" into some Maxforce TMS boxes in areas of the northeastern United States, compromising the child resistant box. Due to this problem, the Federal Environmental Protection Agency (EPA) has asked that all similarly designed TMS boxes applied in 2006 be covered with a protective shroud capable of preventing squirrel damage.
An unusual, organic approach to control of ticks and prevention of Lyme disease involves the use of domesticated guineafowl. Guinea Fowl are voracious consumers of insects and have a particular fondness for ticks. They may reduce dependence on chemical pest-control methods.. Many victims of ticks and others with concern often turn to the Guinea Fowl Breeders Association found at Guinea Fowl Breeders Association for advice on this topic.
A vaccine against a North American strain of the spirochetal bacteria was available between 1998 and 2002. When taking it off the market, the manufacturer cited poor sales, though some people believe that the actual reason was that the vaccine was not safe or effective at all.
The advice of the UK's Hospital for Tropical Diseases is that significant exposure (an attached mite for more than twelve hours) should be managed, as in America & Germany, with Doxycycline 100 mg twice a day for three days. Patients should be advised to report any Erythema migrans over the subsequent two to six weeks. If there should be suspicion of disease, then a course of Doxycycline should be immediately given for ten days; without awaiting serology tests which only yield positive results after an interval of one to two months.
Proper Removal of Ticks
There are many urban legends about the proper and effective method to remove a tick. One legend states that something hot (cigarette; burnt match) should be applied to the back of the tick, which causes the tick to remove its head from the victim. It further states that ticks "screw" their heads into their victims; therefore, one must "unscrew" the head. These legends are incorrect and potentially dangerous. Proper removal of a tick: use a pair of tweezers, grab the head of the tick near the mouth, and pull it out. The area should then be disinfected with rubbing alcohol or hydrogen peroxide. If the head is not completely removed, local infection of the person/animal bitten may result, and a doctor should be consulted (or a veterinarian if the tick was removed from a pet).
Ecology
Urbanization and other anthropogenic factors can be implicated in the spread of the Lyme disease into the human population. In many areas, expansion of suburban neighborhoods has led to the gradual deforestation of surrounding wooded areas and increasing "border" contact between humans and tick-dense areas. Human expansion has also resulted in a gradual reduction of the predators that normally hunt deer as well as mice, chipmunks and other small rodents--the primary reservoirs for Lyme disease. As a consequence of increased human contact with host and vector, the likelihood of transmission to Lyme residents has greatly increased. Researchers are also investigating possible links between global warming and the spread of vector-borne diseases including Lyme disease.
The deer tick (Ixodes scapularis, the primary vector in the northeastern U.S.) has a two-year life cycle, first progressing from larva to nymph, and then from nymph to adult. The tick feeds only once at each stage. In the fall, large acorn forests attract deer as well as mice, chipmunks and other small rodents infected with B. burgdorferi. During the following spring, the ticks lay their eggs. The rodent population then "booms." Tick eggs hatch into larvae, which feed on the rodents; thus the larvae acquire infection from the rodents. (Note: At this stage, it is proposed that tick infestation may be controlled using acaricides ( miticide). A commercial method is to provide nesting material soaked in permethrin ( Damminix).) The infected larvae molt into nymphs. These infected nymphs transmit the majority of Lyme infection to humans, feeding on humans and small animals from spring through summer. The nymphs then molt into adults, which feed on larger animals such as deer in the fall and early spring. Adult ticks may also transmit disease to humans. After feeding, female adult ticks lay their eggs on the ground, and the cycle is complete. Note: on the west coast, Lyme disease is spread by the western black-legged tick (Ixodes pacificus), which has a different life cycle.
The risk of acquiring Lyme disease does not necessarily depend on the existence of a local deer population, as is commonly assumed. New research suggests that eliminating deer from smaller areas (less than 2.5 ha or 6.2 acres) may in fact lead to an increase in tick density and the rise of "tick-borne disease hotspots".
Epidemiology
The number of reported cases of the disease have been increasing, as are endemic regions in North America. For example, it had previously been accepted that Borrelia burgdorferri couldn't be maintained in an enzootic cycle in the Southern United States because it was assumed the large lizard population would dilute the prevalence of Borrelia burgdorferri in local tick poplations. The reason this assumption was made was based upon a study which found that lizard blood from certain species was lethal to Borrelia burgdorferri. Secondly, in areas where lizards are abundant, they are often used as blood meals by sequestering ticks. However, when this theory has been examined it has failed to be as promising in the real world as it had been in previous lab experiments. This suggests that the enzootic cycle in areas of the country other than New England are highly complex and the study needed to identify risk factors will be a difficult epidemiological task. For example, in recent studies from Clark, results have shown that the prevalence of Borrelia burgdorferri has been very high, even among lizards. The author speculated that the enzootic cycle in nature for Borrelia burgdorferri in the South was quite different from that found in New England. For instance, in repeated studies from Clark, a high prevalence of Borrelia burgdorferri sensu lato was found in her study of Southern enzootic cycles of Borrelia burgdorferri, whereas in New England, enzootic cycles are almost entirely Borrelia burgdorferri sensu stricto. Lyme disease is reported in nearly every state in the U.S., but there are concentrated areas in the north-east, mid-Atlantic states, Wisconsin, Minnesota, and northern California. Lyme disease is also endemic to Europe and Asia.
History
Lyme disease is named after a cluster of cases that occurred in and around Old Lyme and Lyme, Connecticut in 1975. Before 1975, elements of Borrelia infection were also known as Tickborne meningopolyneuritis, Garin-Bujadoux syndrome, Bannwarth syndrome or sheep tick fever.
The disease was first documented as a skin rash in Europe in 1883. Over the years, researchers there identified additional features of the disease, including an unidentified pathogen, its response to penicillin, the role of the Ixodes tick (black legged tick) as its vector, and other symptoms including those affecting the central nervous system.
In the U.S., Borrelia burgdorferi has been isolated in the skin of white-footed mice in museum specimens that date back to the 1870s in Massachusetts, but researchers were unaware of the organism's existence until the 1970s. Interest in tick-borne infections in the U.S. began with the first report of tick-borne relapsing fever in 1905, and the discovery of the wood tick's role as a vector of Rocky Mountain spotted fever the following year. However, the full syndrome now known as Lyme disease was not recognized until a cluster of cases originally thought to be juvenile rheumatoid arthritis was identified in three towns in southeastern Connecticut in 1977. Two of these towns, Lyme and Old Lyme, gave the disease its popular name.
In 1982 a novel spirochete was isolated and cultured from the midgut of Ixodes ticks, and subsequently from patients with Lyme disease. The infecting agent was first identified by Jorge Benach, and soon after isolated by Willy Burgdorfer, a scientist at the National Institutes of Health, who specialized in the study of spirochete microorganisms. The spirochete was named Borrelia burgdorferi in his honour. Burgdorfer was the partner in the successful effort to culture the spirochete, along with Alan Barbour.
In Europe, the earliest known cases of Lyme disease date back about 30 years. However, the disease was not thoroughly recognized before 1998. Patients entering doctor's offices with vague symptoms such as chronic exhaustion and joint pains where often wrongly diagnosed. Fortunately, more knowledge of the disease and its treatment is available now, and many patients are treated with antibiotics on time to prevent serious infection. However, many people in countries such as The Netherlands and France were diagnosed too late and still suffer from the disease in spite of regular antibiotics treatment.