Crystallographic defects in diamond
2007 Schools Wikipedia Selection. Related subjects: Materials science
Crystallographic defects in the crystal lattice of diamond are common; they may be the result of extrinsic substitutional impurities, or intrinsic (interstitial and structural) anomalies. All diamonds possess crystal lattice defects of some sort; the defects themselves may be either anthropogenic or natural, epigenetic or syngenetic. The material properties of diamond are affected by these defects and determine to which type a diamond is assigned; the most dramatic effects are on a diamond's colour and semiconductivity, as explained by the band theory.
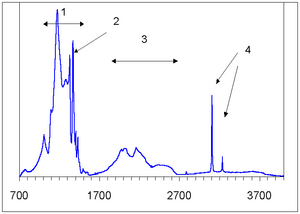
The defects can be detected by different types of spectroscopy, including ESR, photoluminescence in ultraviolet light, and absorption of infrared light. The resulting absorption spectrum can then be analyzed, identified, and used to separate natural from synthetic or enhanced diamonds.
Extrinsic defects
The burning of diamonds in a vacuum and the analysis of resultant gases and remnant matter has shown that diamonds can contain many elements present as substitutional (i.e., replacing carbon atoms in the lattice) impurities: nitrogen, boron, hydrogen, oxygen, sulfur, nickel, cobalt, and iron have all been thus detected.
Nitrogen
The most common impurity in diamond is nitrogen, which can comprise up to 1 % of a diamond by mass. Nitrogen as a diamond impurity was first identified in 1959 by Kaiser and Bond of Bell Telephone (Kaiser and Bond 1959). Previously, all lattice defects in diamond were thought to be the result of structural anomalies; later research revealed nitrogen to be present in most diamonds and in many different configurations.
The light absorption and other material properties of diamond are highly dependent upon nitrogen content and aggregation state. Although all aggregate configurations cause absorption in the infrared and ultraviolet, diamonds with high levels of nitrogen are usually colorless. It is the interactions between different aggregate configurations which cause colour rather than the aggregates themselves (Anderson et. al. 1998, p. 215).
Main nitrogenous defects
There are more than 50 forms of nitrogenous defects that occur in diamonds, and the three main forms observed in visible and infrared spectra are as follows:
- C centre
- C centre defects consist of single substitutional nitrogen atoms in the diamond lattice that are spacially isolated. These are easily seen in ESR spectra (in which they are called P1 centers). C form defects impart a deep yellow to brown colour; these diamonds are classed Type Ib and are commonly known as canary diamonds, which are rare in gem form. In most cases synthetic diamonds contain a high level of nitrogen in the C form because nitrogen from the atmosphere is difficult to exclude from the synthesis process; as little as one nitrogen atom per 100,000 carbon atoms will produce a deep yellow (Nassau 1980, p. 191). Because the nitrogen atoms have five available electrons (one more than the carbon atoms they replace), they act as deep donors; that is, each substituting nitrogen has an extra electron to donate, thereby forming a donator energy level within the band gap. Light with energy above ca. 2.5 eV and above can excite the donor electrons into the conduction band, thereby allowing light absorption (Nassau, p. 332).
- A centre
- The A center is probably the most common defect in natural diamonds. The structure of this form remains a topic of debate: first researchers supposed that it consisted of nitrogen, but later the conclusion was reached that the A center was due to microscopic platelets (now platelets connected with B2 peaks). This theory remained for twenty years, until E. V. Sobolev offered the theory of two nitrogen atoms (bonded strongly together as a molecular pair) replacing carbon in the diamond lattice. Recent research has shown the accuracy of this model. The A centre does not cause discoloration on its own; these diamonds are classed as Type IaA.
- B1 centre
- The structure of B1 defects is not yet clear. The most popular explanation involves four nitrogen atoms surrounding a vacancy. These diamonds are classed as Type IaB; most gem diamonds contain a mixture of A center and B centre defects, together with N3 centers, the combination producing the yellow-brown Cape series. As with A center defects, B1 centre defects do not cause discoloration by themselves (Anderson et. al., p. 215).
Minor nitrogenous defects
- N3 centre
- The N3 centre consists of three nitrogen atoms surrounding a vacancy in a flat configuration. It can occur along with other aggregate forms, with which it produces strong colors—particularly with A and B1 centers (Anderson et. al., p. 215). The N3 centre is paramagnetic so its structure is well-developed by the ESR method. In ultraviolet fluorescence spectra, this defect produces a characteristic absorption line in the far violet at 415.5 nm, termed the N3 line (O'Donoghue 2002, p. 52). A closely related aggregate is the N2 centre, which produces a line at 478 nm (Reinitz 2005).
Boron
Diamonds containing boron as a substitutional impurity are termed Type IIb. Only one percent of diamonds are of this type, and most are blue to grey (O'Donoghue 2002, p. 52). The boron acts as an acceptor; that is, because the substituting boron atoms have one less available electron than the carbon atoms they replace, each boron atom creates an electron hole in the band gap that can accept an electron from the valence band. This allows red light absorption, and due to the small energy (c. 0.4 eV) needed for the electron to leave the valence band, holes are created in the latter even via thermal heat at room temperatures. These holes can move in an electric field and render the diamond electrically conductive (i.e., a p-type semiconductor). Very little substitutional boron is required for this to happen—a typical ratio is one boron atom per 1,000,000 carbon atoms (Nassau, p. 333).
Type IIb diamonds transmit in the ultraviolet down to c. 250 nm but do not absorb in the visible region apart from the far red (hence the blue colour); they may phosphoresce blue after exposure to shortwave ultraviolet. Synthetic diamonds containing boron are blue and either Type IIb or a mixture of IIb and IIa material (O'Donoghue 2002, p. 52, 46).
Intrinsic defects
Every natural diamond crystal contains typical intrinsic or self-defects: vacancies, dislocations, and interstitial atoms.
Vacancies
A vacancy is an empty position in a diamond's lattice. Vacancies may be affected or created by radiation damage—high-energy subatomic particles knock carbon atoms out of the lattice. This may be the result of natural or artificial radiation (see Diamond enhancement - Irradiation). The vacancies interact with interstitial atoms ("extra" atoms, most commonly nitrogen, which occupy space between carbon atoms rather than substituting for them) and act as colour centers by absorbing visible light, thus producing green or blue colors in Type I, and brown colors in Type IIa diamond. Radiation-induced vacancies can be detected by ultraviolet fluorescence, as well as by a characteristic absorption line at 741.2 nm, termed the GR 1 (General Radiation) line. This line is destroyed if the diamond is annealed above 400°C, after which a number of additional lines (e.g. 575, 595, 503 [H3 center], 497, 1935 [H1c center], and 2924 [H1b centre] nm) are formed (Gemlab 2002b).
The annealing process (or the heat of the earth over geological timescales) also allows carbon atoms neighboring a vacancy to jump into a vacant place and leave an empty position in the diamond lattice; by this process a vacancy can migrate through the diamond, and can form compound defects with other vacancies, interstitial atoms (forming Frenkel pairs), or nitrogenous defects (NV centers). The newly-formed compound defects are optically active, producing strong yellows, pinks, and reds, the precise colour dependent on the annealing time and type of pre-existing defects present. Vacancies can also be created or modified by HTHP treatment.
Dislocations
The purest diamonds, which contain little if any extrinsic impurities (Type IIa), may have their colour modified by structural dislocations or plastic deformations, which are breaks in the translational symmetry of the lattice. There are two important types of dislocations in diamond: the glide set, in which bonds break between layers of atoms with different indices (those not lying directly above each other); and the shuffle set, in which the breaks occur between atoms of the same index. The dislocations produce dangling bonds which introduce energy levels into the band gap, enabling the absorption of light (Kolodzie and Bleloch).
These defects are thus believed to cause brown, pink, or purple coloration. Like boron-containing Type IIb diamonds, Type IIa diamonds transmit in the ultraviolet down to 250 nm. If treated with high temperatures and high pressures, the dislocations can be "healed" and the colour removed (see next section).
- B2 centre
- Some diamonds contain platelets in the 100 plane visible by microscope. This intriguing defect causes a sharp peak at 1600 cm-1 in IR spectra.
Effects of HTHP on defects
Experiments with synthetic and natural diamonds treated at high temperatures (1700–2800°C) and high pressures (6–8 GPa; HTHP) have shown that, with time, lattice defects can be altered or repaired. In Type IIa diamonds with structural dislocations, a small number of NV centers are created—as indicated by absorption peaks at 637 nm (NV-), 575 (NV0), and 3760 cm-1—the lattice is realigned and ruptured bonds repaired, and much of the original brown color is removed (O'Donoghue and Joyner 2003, p. 35; Gemlab 2002a). Sometimes, a pink color is induced instead; some are left with a yellowish cast due to the NV centers. The occasional brown Type IIb diamonds subjected to HTHP will turn pure blue due to their boron content. HTHP can also be used to remove color from brown Type IaB diamonds colored by grainig planes which contain amorphous carbon. No single nitrogen is introduced in this case; however, N3 centers sometimes are, and impart a light yellow-grey colour (Deljanin and Fritsch 2000).
Pale yellow Type IaA/B Cape series diamonds can have their A- and B-centers converted (broken up) to C-centers via HTHP above 1960°C, thereby creating intense canary-type colors in shades of yellow, brownish-yellow, olive, or green. The strongest colors are produced at the highest temperatures, which also produces tell-tale green transmission fluorescence under visible and ultraviolet light (Gemlab 2002b). The green fluorescence is attributed to the H3 center (a vacancy trapped at an A-center aggregate) and produces a line at 503 nm. Green HTHP-treated diamonds also exhibit a line at 985 (or 986) nm, known as the H2 centre, that is also the result of a vacancy-nitrogen aggregate complex (Reinitz 2005). The H1c and H1b centers common to irradiated and annealed diamonds may also be present.
Diamonds with C form aggregates can be converted to the A form. This process is called aggregation of nitrogen because nitrogen atoms tend to assemble to the aggregate locations with lower energy. The next step of this process is conversion of A form to B1 form of nitrogen with attendant constitution of platelets (B2 centre). Possibly when most of the nitrogen is in B1 form the platelets disintegrate with the formation of micro-voids. Conversion of A form into B1 form takes place at noticeably higher temperatures and/or longer treatment times.