Cerebellum
2007 Schools Wikipedia Selection. Related subjects: General Biology
The cerebellum (Latin: "little brain") is a region of the brain that plays an important role in the integration of sensory perception and motor output. Many neural pathways link the cerebellum with the motor cortex—which sends information to the muscles causing them to move—and the spinocerebellar tract—which provides feedback on the position of the body in space ( proprioception). The cerebellum integrates these pathways, using the constant feedback on body position to fine-tune motor movements.
Because of this 'updating' function of the cerebellum, lesions within it are not so debilitating as to cause paralysis, but rather present as feedback deficits resulting in disorders in fine movement, equilibrium, posture, and motor learning. Initial observations by physiologists during the 18th century indicated that patients with cerebellar damage show problems with motor coordination and movement. Research into cerebellar function during the early to mid 19th century was done via lesion and ablation studies in animals. Research physiologists noted that such lesions led to animals with strange movements, awkward gait, and muscular weakness. These observations and studies led to the conclusion that the cerebellum was a motor control structure. However, modern research shows that the cerebellum has a broader role in a number of key cognitive functions, including attention and the processing of language, music, and other sensory temporal stimuli.
General features
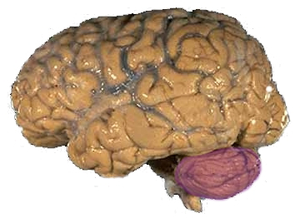
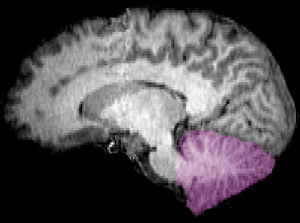
The cerebellum is located in the inferior posterior portion of the head (the hindbrain), directly dorsal to the pons, and inferior to the occipital lobe (Figs. 1 and 3). Because of its large number of tiny granule cells, the cerebellum contains nearly 50% of all neurons in the brain, although it constitutes only 10% of total brain volume. The cerebellum receives nearly 200 million input fibers; in contrast, the optic nerve is composed of a mere one million fibers.
The cerebellum is divided into two large hemispheres, much like the cerebrum, and contains ten smaller lobules. The cytoarchitecture (cellular organization) of the cerebellum is highly uniform, with connections organized into a rough, three-dimensional array of perpendicular circuit elements. This organizational uniformity makes the nerve circuitry relatively easy to study. To envision this "perpendicular array", one might imagine a tree-lined street with wires running straight through the branches of one tree to the next.
Development and evolution
During the early stages of embryonic development, the brain starts to form in three distinct segments: the prosencephalon, mesencephalon, and rhombencephalon. The rhombencephalon is the most caudal (toward the tail) segment of the embryonic brain; it is from this segment that the cerebellum develops. Along the embryonic rhombencephalic segment develop eight swellings, called rhombomeres. The cerebellum arises from two rhombomeres located in the alar plate of the neural tube, a structure that eventually forms the brain and spinal cord. The specific rhombomeres from which the cerebellum forms are rhombomere 1 (Rh.1) caudally (near the tail) and the "isthmus" rostrally (near the front).
The neural tube is organized so that the alar plate typically gives rise to structures involved in sensory functions; the basal (ventral, or lower) plate gives rise to motor functioning structures. Given its alar plate origins, the cerebellum would be expected to be devoted primarily to sensory functions. Despite its embryological origin, one of the many ironies of the cerebellum is that it functions primarily to modulate motor function.
Two primary regions are thought to give rise to the neurons that make up the cerebellum. The first region is the ventricular zone in the roof of the fourth ventricle. This area produces Purkinje cells and deep cerebellar nuclear neurons. These cells are the primary output neurons of the cerebellar cortex and cerebellum. The second germinal zone (cellular birthplace) is known as the external granular layer. This layer of cells—found on the exterior the cerebellum—produces the granule neurons. Once born, the granule neurons migrate from this exterior layer to form an inner layer known as the internal granule layer. The external granular layer ceases to exist in the mature cerebellum, leaving only granule cells in the internal granule layer. The cerebellar white matter may be a third germinal zone in the cerebellum; however, its function as a germinal zone is controversial.
The cerebellum is of archipalliar phylogenetic origin. The pallium is a term for gray matter that forms the cortex. The archipallium is the one of the most evolutionarily primitive brain regions. The circuits in the cerebellar cortex look similar across all classes of vertebrates, including fish, reptiles, birds, and mammals (e.g., Fig. 2). This has been taken as evidence that the cerebellum performs functions important to all vertebrate species.
Anatomy
The cerebellum contains similar gray and white matter divisions as the cerebrum. Embedded within the white matter—which is known as the arbor vitae ( Tree of Life) in the cerebellum due to its branched, treelike appearance—are four deep cerebellar nuclei. Three gross phylogenetic segments are largely grouped by general function. The three cortical layers contain various cellular types that often create various feedback and feedforward loops. Oxygenated blood is supplied by three arterial branches off the basilar and vertebral arteries.
Divisions
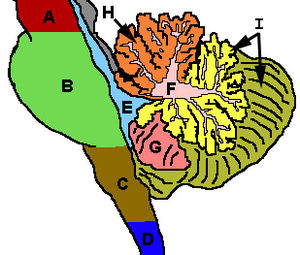
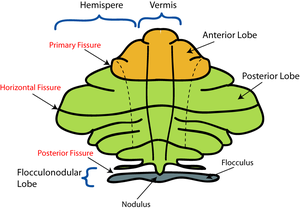
There are three phylogenetic divisions within the cerebellum: the flocculonodular, anterior, and posterior lobes (Fig. 3). These divisions are also called the archicerebellum, paleocerebellum, and neocerebellum, respectively, terms that more clearly reflect the method of dividing the lobes by their evolutionary age. The archicerebellum represents the oldest evolutionary cerebellar structure, and the neocerebellum represents the most recently evolved region. These divisions are divided from the front to the back of the cerebellum; starting with the flocculonodular lobe in the front, and ending with the posterior lobe in the back. The anterior and posterior lobes are separated by the primary fissure. The posterior and flocculonodular lobes are separated by the posterolateral fissure (Fig. 4).
The cerebellum can also be divided by function rather than evolutionary age. This results in three functional divisions that run perpendicular to the previously mentioned phylogenetic divisions. Rather than running from front to back, like the phylogenetic divisions, the functional regions align from the midline outwards toward the sides of the body. The midline division is called the medial zone, also known as the cerebellar vermis (“worm”) because of its long, slender shape; the vermis is further subdivided into smaller regions called lobules. The region just lateral (away from the centre) of the vermis is called the intermediate zone; the most lateral, outer region is the lateral zone (Fig. 4).
Much of what is understood about the functions of the cerebellum stems from careful documentation of the effects of focal lesions in human patients who have suffered from injury or disease or through animal lesion research.
Cerebellar structure and function from a phylogenetic perspective
Archicerebellum
The archicerebellum is associated with the flocculonodular lobe and is mainly involved in balance ( vestibular system) and eye movement functions. It receives input from the inferior and medial vestibular nuclei and sends fibers back to the vestibular nuclei, creating a feedback loop that allows for the constant maintenance of balance.
Paleocerebellum
The paleocerebellum controls proprioception related to muscle tone (constant, partial muscle contraction that is important for the maintenance of posture). The paleocerebellum receives its inputs from the dorsal and ventral spinocerebellar tracts, which carry information about the position and forces acting on the legs. The paleocerebellum then sends axonal projections to the deep cerebellar nuclei.
Neocerebellum
The neocerebellum receives input from the pontocerebellar tract and projects to the deep cerebellar nuclei. The pontocerebellar tract originates at the pontine nuclei, which receive their input from the cerebral motor cortex. Thus, the neocerebellum is associated with motor control, in particular, the coordination of fine finger movements such as those required by typing.
The functional organization of the cerebellum
Vermis
The vermis receives its inputs mainly from the spinocerebellar tracts from the trunk of the body. These tracts carry to the vermis information on the position and balance of the torso. The vermis sends projections to the fastigial nucleus of the cerebellum, which then sends output to the vestibular nuclei. The vestibular nuclei are structures important for the maintenance of balance.
Intermediate zone
The intermediate zone (or paravermis) receives input from the corticopontocerebellar fibers that originate from the motor cortex. These fibers carry a duplicate of the information that was sent from the motor cortex to the spine in order to effect a movement. The intermediate zone also receives sensory feedback from the muscles. These two streams of information are integrated by this region, allowing for the feedback comparison of what the muscles are supposed to be doing with what they are actually doing.
Lateral zone
The lateral zone receives input from the parietal cortex via pontocerebellar mossy fibers regarding the location of the body in the world. The large numbers of feedback circuits allow for the integration of this body position information with indications of muscle position, strength, and speed.
Deep nuclei
The four deep cerebellar nuclei are in the centre of the cerebellum, embedded in the white matter. These nuclei receive inhibitory ( GABAergic) inputs from Purkinje cells in the cerebellar cortex and excitatory ( glutamatergic) inputs from mossy fibre pathways. Most output fibers of the cerebellum originate from these nuclei. One exception is that fibers from the flocculonodular lobe synapse directly on vestibular nuclei without first passing through the deep cerebellar nuclei. The vestibular nuclei in the brainstem are analogous structures to the deep nuclei, since they receive both mossy fibre and Purkinje cell inputs.
From lateral to medial, the four deep cerebellar nuclei are the dentate, emboliform, globose, and fastigial. An easy mnemonic device to remember these names and positions relative to their position from the midline is the phrase "Don't Eat Greasy Food", where each letter indicates the lateral to medial location in the cerebellar white matter. Some animals do not have distinct emboliform and globose nuclei, instead having a single, fused nucleus interpositus (interposed nucleus). In animals with distinct emboliform and globose nuclei, the term interposed nucleus is often used to refer collectively to these two nuclei.
In general, each pair of deep nuclei is associated with a corresponding region of cerebellar surface anatomy. The dentate nuclei are deep within the lateral hemispheres, the interposed nuclei are located in the paravermal (intermediate) zone, and the fastigial nuclei are in the vermis. These structural relationships are generally maintained in the neuronal connections between the nuclei and associated cerebellar cortex, with the dentate nucleus receiving most of its connections from the lateral hemispheres, the interposed nuclei receiving inputs mostly from the paravermis, and the fastigial nucleus receiving primarily afferents from the vermis.
Cortical layers
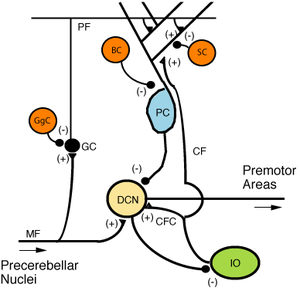
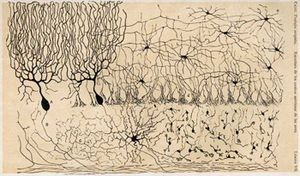
There are three layers to the cerebellar cortex; from outer to inner layer, these are the molecular, Purkinje, and granular layers. The function of the cerebellar cortex is essentially to modulate information flowing through the deep nuclei. The microcircuitry of the cerebellum is schematized in Figure 5. Mossy and climbing fibers carry sensorimotor information into the deep nuclei, which in turn pass it on to various premotor areas, thus regulating the gain and timing of motor actions. Mossy and climbing fibers also feed this information into the cerebellar cortex, which performs various computations, resulting in the regulation of Purkinje cell firing. Purkinje neurons feed back into the deep nuclei via a potent inhibitory synapse. This synapse regulates the extent to which mossy and climbing fibers activate the deep nuclei, and thus control the ultimate effect of the cerebellum on motor function. The synaptic strength of almost every synapse in the cerebellar cortex has been shown to undergo synaptic plasticity. This allows the circuitry of the cerebellar cortex to continuously adjust and fine-tune the output of the cerebellum, forming the basis of some types of motor learning and coordination. Each layer in the cerebellar cortex contains the various cell types that comprise this circuitry.
Granular layer
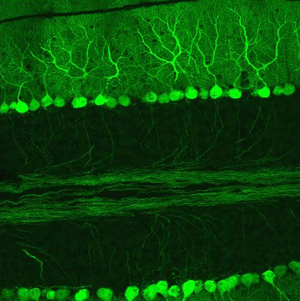
The innermost layer contains the cell bodies of two types of cells: the numerous and tiny granule cells, and the larger Golgi cells. Mossy fibers enter the granular layer from their main point of origin, the pontine nuclei. These fibers form excitatory synapses with the granule cells and the cells of the deep cerebellar nuclei. The granule cells send their T-shaped axons—known as parallel fibers—up into the superficial molecular layer, where they form hundreds of thousands of synapses with Purkinje cell dendrites. The human cerebellum contains on the order of 60 to 80 billion granule cells, making this single cell type by far the most numerous neuron in the brain (roughly 70% of all neurons in the brain and spinal cord, combined). Golgi cells provide inhibitory feedback to granule cells, forming a synapse with them and projecting an axon into the molecular layer.
Purkinje layer
The middle layer contains only one type of cell body—that of the large Purkinje cell. Purkinje cells are the primary integrative neurons of the cerebellar cortex and provide its sole output. Purkinje cell dendrites are large arbors with hundreds of spiny branches reaching up into the molecular layer (Fig. 6). These dendritic arbors are flat—nearly all of them lie in planes—with neighboring Purkinje arbors in parallel planes. Each parallel fibre from the granule cells runs orthogonally through these arbors, like a wire passing through many layers. Purkinje neurons are GABAergic—meaning they have inhibitory synapses—with the neurons of the deep cerebellar and vestibular nuclei in the brainstem. Each Purkinje cell receives excitatory input from 100,000 to 200,000 parallel fibers. Parallel fibers are said to be responsible for the simple (all or nothing, amplitude invariant) spiking of the Purkinje cell.
Purkinje cells also receive input from the inferior olivary nucleus via climbing fibers. A good mnemonic for this interaction is the phrase "climb the olive tree", given that climbing fibers originate from the inferior olive. Each Purkinje cell receives input from a single climbing fibre in the form of a powerful excitatory signal. These action potentials are of a rare class in that the excitatory postsynaptic response from climbing fibers are amplitude variant. These responses are known as complex spikes.
Molecular layer
This outermost layer of the cerebellar cortex contains two types of inhibitory interneurons: the stellate and basket cells. It also contains the dendritic arbors of Purkinje neurons and parallel fibre tracts from the granule cells. Both stellate and basket cells form GABAergic synapses onto Purkinje cell dendrites.
Peduncles
Similarly, the cerebellum follows the trend of "threes", with three major input and output peduncles (fibre bundles). These are the superior (brachium conjunctivum), middle (brachium pontis), and inferior (restiform body) cerebellar peduncles. There are three sources of input to the cerebellum, in two categories consisting of mossy and climbing fibers, respectively. Mossy fibers can originate from the pontine nuclei, which are clusters of neurons located in the pons that carry information from the contralateral cerebral cortex. They may also arise within the spinocerebellar tract whose origin is located in the ipsilateral spinal cord. Most of the output from the cerebellum initially synapses onto the deep cerebellar nuclei before exiting via the three peduncles. The most notable exception is the direct inhibition of the vestibular nuclei by Purkinje cells.
Superior cerebellar peduncle
While there are some afferent fibers from the anterior spinocerebellar tract that are conveyed to the anterior cerebellar lobe via this peduncle, most of the fibers are efferents. Thus, the superior cerebellar peduncle is the major output pathway of the cerebellum. . Most of the efferent fibers originate within the dentate nucleus which in turn project to various midbrain structures including the red nucleus, the ventral lateral/ventral anterior nucleus of the thalamus, and the medulla. The dentatorubrothalamocortical (dentate nucleus > red nucleus > thalamus > premotor cortex) and cerebellothalamocortical (cerebellum > thalamus > premotor cortex) pathways are two major pathways that pass through this peduncle and are important in motor planning.
Middle cerebellar peduncle
This is composed entirely of afferent fibers originating within the pontine nuclei as part of the massive corticopontocerebellar (cerebral cortex > pons > cerbellum) tract. These fibers descend from the sensory and motor areas of the cerebral neocortex and make the middle cerebellar peduncle the largest of the three cerebellar peduncles.
Inferior cerebellar peduncle
This carries many types of input and output fibers that are mainly concerned with integrating proprioceptive sensory input with motor vestibular functions such as balance and posture maintenance. Proprioceptive information from the body is carried to the cerebellum via the dorsal spinocerebellar tract. This tract passes through the inferior cerebellar peduncle and synapses within the paleocerebellum. Vestibular information projects onto the archicerebellum. This peduncle also carries information directly from the Purkinje cells to the vestibular nuclei in the dorsal brainstem located at the junction between the pons and medulla.
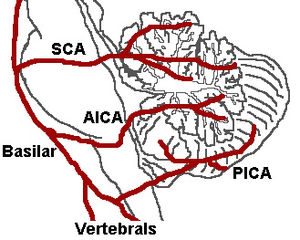
Blood supply
Three arteries supply blood to the cerebellum (Fig. 7): the superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), and posterior inferior cerebellar artery (PICA).
Superior cerebellar artery
The SCA branches off the lateral portion of the basilar artery, just inferior to its bifurcation into the posterior cerebral artery. Here it wraps posteriorly around the pons (to which it also supplies blood) before reaching the cerebellum. The SCA supplies blood to most of the cerebellar cortex, the cerebellar nuclei, and the middle and superior cerebellar peduncles.
Anterior inferior cerebellar artery
The AICA branches off the lateral portion of the basilar artery, just superior to the junction of the vertebral arteries. From its origin, it branches along the inferior portion of the pons at the cerebellopontine angle before reaching the cerebellum. This artery supplies blood to the anterior portion of the inferior cerebellum, and to the facial (CN VII) and vestibulocochlear nerves (CN VIII).
Obstruction of the AICA can cause paresis, paralysis, and loss of sensation in the face; it can also cause hearing impairment.
Posterior inferior cerebellar artery
The PICA branches off the lateral portion of the vertebral arteries just inferior to their junction with the basilar artery. Before reaching the inferior surface of the cerebellum, the PICA sends branches into the medulla, supplying blood to several cranial nerve nuclei. In the cerebellum, the PICA supplies blood to the posterior inferior portion of the cerebellum, the inferior cerebellar peduncle, the nucleus ambiguus, the vagus motor nucleus, the spinal trigeminal nucleus, the solitary nucleus, and the vestibulocochlear nuclei.
Dysfunction
Patients with cerebellar dysfunction experience problems in walking, balance, and accurate hand and arm movement. Recent brain imaging studies using functional magnetic resonance imaging (fMRI) show that the cerebellum is important for language processing and selective attention. Neuropsychiatric disorders such as dyslexia, schizophrenia and autism appear to be associated with a deficiency in the cerebellum, which may also play a role in the development of certain ataxias, including a form of cerebral palsy. Spinocerebellar ataxia patients suffer cerebellar degeneration. It is believed that opsoclonus myoclonus syndrome is caused by an autoimmune attack on the cerebellum among other brain regions.
Lesions of the cerebellum
Patients with cerebellar lesions (injuries) typically exhibit deficits during movement execution. For example, they show "intention tremors"—a tremor occurring during movement rather than at rest, as seen in Parkinson's disease. Patients may also show dysmetria, i.e., an overestimation or underestimation of force, resulting in overshoot or undershoot when reaching for a target. Another common sign of cerebellar damage is an inability to perform rapid alternating movements.
The anterior and medial aspects of the cerebellum represent information ipsilaterally; thus, damage to this region on one side affects the movement on the same side of the body. The posterior and lateral aspects of the cerebellum represent information bilaterally; damage to this region has been shown to impair sensory–motor adaptation, while leaving motor control unaffected. In certain instances, a patient experiences a focal lesion. Such localized lesions cause a wide variety of symptoms related to their location in the cerebellum. A striking example is archicerebellar lesions, which cause motor symptoms not unlike those seen during intoxication: uncoordinated movements, swaying, unstable walking, and a wide gait. To avoid suspicion by the police of public drunkenness, American patients who suffer archicerebellar lesions carry identification cards written by their physicians, indicating their medical condition.
A lesion to the paleocerebellum causes severe disturbance in muscle tone and bodily posture, resulting in weakness to the side of the body opposite the lesion. A neocerebellar lesion is associated with deficits in skilled voluntary movement, such as playing the piano. A lesion to the intermediate zone causes problems with fine-tuning and corrective movements. Patients with this type of lesion who hold their fingers in front of them have great difficulty in moving those fingers together. Patients with a lesion to the lateral zone have difficulty in controlling fine muscle movements and exhibit symptoms similar to those of patients with an intermediate zone lesion.
Alcohol abuse is also a common cause of cerebellar lesions. Alcohol abuse can lead to thiamine deficiency, which in the cerebellum will cause degeneration of the anterior lobe. This degeneration leads to a wide, staggering gait but does not affect arm movement or speech.
Ischemia and thrombosis
An obstruction of the posterior inferior cerebellar artery (known as 'PICA syndrome') can cause a wide range of characteristic effects. An obstruction of the artery causes cell death as the cells are deprived of oxygen and nutrients provided by the blood. PICA syndrome manifests as a loss of sensation in the contralateral limbs due to damage of the inferior cerebellar peduncle as well as dizziness and nausea due to loss of blood to the nucleus ambiguus and vestibulocochlear nuclei.
Theories about cerebellar function
Three main theories address the function of the cerebellum. One claims that the cerebellum functions as a regulator of the "timing of movements". This has emerged from studies of patients whose timed movements are disrupted. The second theory claims that the cerebellum operates as a learning machine, encoding information as does a computer. This was first proposed by Marr and Albus in the early 1970s. The third, "Tensor Network Theory" provides a mathematical model of transformation of sensory (covariant) space-time coordinates into motor (contravariant) coordinates by cerebellar neuronal networks.
Like many controversies in the physical sciences, there is evidence supporting the above hypotheses. Studies of motor learning in the vestibulo-ocular reflex and eyeblink conditioning demonstrate that the timing and amplitude of learned movements are encoded by the cerebellum. Many synaptic plasticity mechanisms have been found throughout the cerebellum. The Marr-Albus model mostly attributes motor learning to a single plasticity mechanism: the long-term depression of parallel fibre synapses. The Tensor Network Theory of sensorimotor transformations by the cerebellum has also been experimentally supported.
With the advent of more sophisticated neuroimaging techniques such as positron emission tomography (PET), and fMRI, numerous diverse functions are now at least partially attributed to the cerebellum. What was once thought to be primarily a motor/sensory integration region is now proving to be involved in many diverse cognitive functions. Paradoxically, despite the importance of this region and the heterogeneous role it plays in motor and sensory functions, people who have lost their entire cerebellum through disease, injury, or surgery can live reasonably normal lives.
Cerebellar modeling
As mentioned in the preceding section, there have been many attempts to model the cerebellar function. The insights provided by the models have also led to extrapolations in the domains of artificial intelligence methodologies, especially neural networks. Some of the notable achievements have been Cerebellatron , Cerebellar Model Associative Memory or CMAC networks, and Spikeforce for robotic movement control, and "Tensor Network Theory".