Thalassemia
2007 Schools Wikipedia Selection. Related subjects: Health and medicine
| ICD- 10 | D 56. |
|---|---|
| ICD- 9 | 282.4 |
Thalassemia (American English) or thalassaemia (British English) is an inherited disease of the red blood cells. In thalassemia, the genetic defect results in reduced rate of synthesis of normal globin chains(c.f. hemoglobinopathy which is a structural change in a globin chain leading to instability or abnormal with the Mediterranean seaamong different populations.
Classification
The thalassemias are classified according to which chain of the globin molecule is affected: in α thalassemia, the production of α globin is deficient, while in β thalassemia the production of β globin is defective. Thalassemia produces a deficiency of α or β globin, unlike sickle-cell disease which produces a specific mutant form of β globin.
Prevalence
The estimated prevalence is 16% in people from Cyprus, 3-14 % in Thailand, and 3-8 % in populations from India, Pakistan, Bangladesh, and China. A lower prevalence has been reported from black people in Africa (0.9%) and northern Europe (0.1%).(4)
Alpha (α) thalassemias
The alpha thalassemias involve the genes HBA1 ( Mendelian Inheritance in Man (OMIM) 141800) and HBA2 ( Mendelian Inheritance in Man (OMIM) 141850), inherited in a Mendelian recessive fashion. It is also connected to the deletion of the 16p chromosome. α thalassemias result in excess β chain production in adults and excess γ chains in newborns. The excess β chains form unstable tetramers that have abnormal oxygen dissociation curves.
There are four genetic loci for α globin. The more of these loci that are deleted or affected by mutation, the more severe will be the manifestations of the disease:
- If all four loci are affected, the fetus cannot live once outside the uterus: most such infants are dead at birth with hydrops fetalis, and those who are born alive die shortly after birth. They are edematous and have little circulating hemoglobin, and the hemoglobin that is present is all tetrameric γ chains (hemoglobin Barts). Usually, this involves homozygous inheritance of an alpha thalassemia trait, type 1.
- If three loci are affected, Hemoglobin H disease results. Two unstable hemoglobins are present in the blood, both hemoglobin Barts (tetrameric γ chains) and hemoglobin H (tetrameric β chains). There is a microcytic hypochromic anaemia with target cells and Heinz bodies (precipitated Hb H) on the peripheral blood smear. The disease may first be noticed in childhood or in early adult life, when the anaemia and splenomegaly are noted. This is usually due to compound heterozygous inheritance of alpha thalassemia type 1 and type 2 traits.
- If two of the four α loci are affected, alpha thalassemia trait, type 1 results. Two α loci permit nearly normal erythropoiesis, though there is a mild microcytic hypochromic anaemia. There is a high prevalence (about 30%) of deletion of one of the two α loci on chromosomes of people of recent African origin, and so the inheritance of two such chromosomes is not uncommon. The disease in this form can be mistaken for iron deficiency anaemia and treated inappropriately with iron. Two modes of alpha thalassemia trait, type 1 has been noted. One involves cis deletion of two alpha loci on the same chromosome; another involves trans deletion of allelelic genes on homologous chromosomes (no. 16).
- If one of the four α loci is affected, alpha minor or alpha+ thalassemia trait or alpha thalassemia trait, type 2 results and there is minimal effect. Three α-globin loci are enough to permit normal hemoglobin production, and there is no anaemia or hypochromia in these people. They have been called α thalassemia carriers.
Beta (β) thalassemias
Beta thalassemia (also known as Cooley's Anaemia) is due to mutations in the HBB gene on chromosome 11 ( Mendelian Inheritance in Man (OMIM) 141900), also inherited in a Mendelian recessive fashion. In β thalassemia, excess α chains are produced, but these do not form tetramers: rather, they bind to the red blood cell membranes producing membrane damage, and at high concentrations have the tendency to form toxic aggregates. The severity of the damage depends on the nature of the mutation. Some mutations (βo) prevent any formation of β chains; others (β+) allow some β chain formation to occur. Recently, increasing reports suggest that up to 5% of patients with beta-thalassemias produce fetal hemoglobin (HbF), and use of hydroxyurea also has a tendency to increase the production of HbF, by as yet unexplained mechanisms.
Any given individual has two β globin alleles:
- If both have thalassemia mutations, a severe microcytic, hypochromic anaemia called β thalassemia major or Cooley's anaemia results. Untreated, this results in death before age twenty: treatment consists of periodic blood transfusion; splenectomy if splenomegaly is present, and treatment of transfusion-caused iron overload. Cure is possible by bone marrow transplantation.
- If only one β globin allele bears a mutation, β thalassemia minor results (sometimes referred to as β thalassemia trait). This is a mild anaemia with microcytosis. Symptoms include weakness and fatigue - in most cases β thalassemia minor may be asymptomatic and many people may be unaware they have this disorder. Detection usually involves counting the mean corpuscular volume (size of red blood cells) and noticing a slightly decreased mean volume than normal.
- Thalassemia intermedia is a condition intermediate between the major and minor forms. Sufferers can often manage a normal life but may need occasional transfusions e.g. at times of illness or pregnancy. This really depends on the severity of their anaemia.
The actual genetic cause of β thalassemias are actually very diverse and a number of different mutations can cause reduced or absent β globin synthesis. Usually, superscripts 0 and + are added to β to indicate complete absence, and deficient synthesis of β globins respectively.
Mainly there are two forms of genetic defects which produce β thalassemias:
- Nondeletion forms: These defects generally involve a single base substitution or small deletion or inserts near or upstream of the β globin gene. Most commonly, mutations occur in the promoter regions preceding the beta-globin genes. Less often, abnormal splice variants are believed to contribute to the disease.
- Deletion forms: Deletions of different sizes involving the β globin gene produce different syndromes such as (βo) or hereditary persistence of fetal hemoglobin syndromes.
Delta (δ) thalassemia
As well as alpha and beta chains being present in hemoglobin about 3% of adult hemoglobin is made of alpha and delta chains. The gene for delta chains is very close to the gene for beta hemoglobin and damage to this gene can also affect the beta chain gene, thus delta thalassemia is usually very similar in effect to Beta thalassemia.
In combination with other hemoglobinopathies
Thalassemia can co-exist with other hemoglobinopathies. The most common of these are:
- hemoglobin E/thalassemia: common in Cambodia, Thailand, and parts of India; clinically similar to β thalassemia major or thalassemia intermedia.
- hemoglobin S/thalassemia, common in African and Mediterranean populations; clinically similar to sickle cell anaemia, with the additional feature of splenomegaly
- hemoglobin C/thalassemia: common in Mediterranean and African populations, hemoglobin C/βo thalassemia causes a moderately severe hemolytic anaemia with splenomegaly; hemoglobin C/β+ thalassemia produces a milder disease.
Treatment and complications
Anyone with thalassemia should consult a properly qualified hematologist.
Thalassemias may co-exist with other deficiencies such as folic acid (or folate, a B-complex vitamin) and iron deficiency (only in Thalassemia Minor).
Thalassemia Minor
Contrary to popular belief, Thalassemia Minor patients should not avoid iron-rich foods by default. A serum ferritin test can determine what their iron levels are and guide them to further treatment if necessary. Thalassemia Minor, although not life threatening on its own, can affect quality of life due to the effects of a mild to moderate anaemia. Studies have shown that thalassemia Minor often coexists with other diseases such as asthma, and even bipolar disorder.
Thalassemia prevention and management
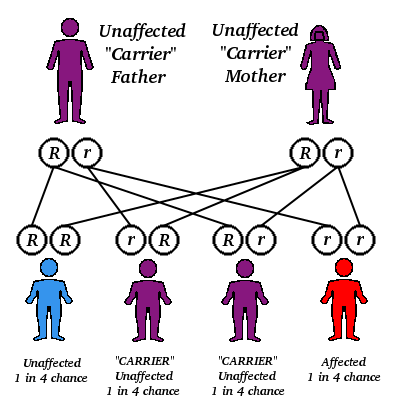
α and β thalassemia are often inherited in an autosomal recessive fashion although this is not always the case. Reports of dominantly inherited α and β thalassemias have been reported the first of which was in an Irish family who had a two deletions of 4 and 11 bp in exon 3 interrupted by an insertion of 5 bp in the β-globin gene. For the autosomal recessive forms of the disease both parents must be carriers in order for a child to be affected. If both parents carry a hemoglobinopathy trait, there is a 25% chance with each pregnancy for an affected child. Genetic counseling and genetic testing is recommended for families that carry a thalassemia trait.
There are an estimated 60-80 million people in the world who carry the beta thalassemia trait alone. This is a very rough estimate and the actual number of thalassemia Major patients is unknown due to the prevalence of thalassemia in less developed countries in the Middle East and Asia. Countries such as India, Pakistan and Iran are seeing a large increase of thalassemia patients due to lack of genetic counseling and screening. There is growing concern that thalassemia may become a very serious problem in the next 50 years, one that will burden the world's blood bank supplies and the health system in general. There are an estimated 1,000 people living with Thalassemia Major in the United States and an unknown number of carriers. Because of the rarity of the disease in countries with little knowledge of thalassemia, access to proper treatment and diagnosis can be difficult.
As with other genetically acquired disorders, aggressive birth screening and genetic counseling is recommended for prevention of a world crisis.
A screening policy exists on both sides of the island of Cyprus to reduce the incidence of thalassemia, which since the program's implementation in the 1970s (which also includes pre-natal screening and abortion) has reduced the number of children born with the hereditary blood disease from 1 out of every 158 births to almost zero.
Benefits
Being a carrier of the disease may confer a degree of protection against malaria, and is quite common among people from Italian or Greek origin, and also in some African and Indian regions. This is probably by making the red blood cells more susceptible to the less lethal species Plasmodium vivax, simultaneously making the host RBC environment unsuitable for the merozoites of the lethal strain Plasmodium falciparum. This is believed to be a selective survival advantage for patients with the various thalassemia traits. In that respect it resembles another genetic disorder, sickle-cell disease.
Epidemiological evidence from Kenya suggests another reason: protection against severe anaemia may be the advantage..
People diagnosed with heterozygous (carrier) Beta-Thalassemia have some protection against coronary heart disease.
Famous people
- Former professional tennis player Pete Sampras is known to be a Thalassemia minor patient.
- Former professional football (soccer) player Zinedine Zidane is known to be a Thalassemia minor patient.
- Rabbi Kohan Shalomim Y. Halahawi, Founder of the African Hebrew Israelite Community Ha' Yisrayli Torah Brith Yahad, and Doctor of Electro-homeopathic Medicine MD(AM), Edenic-Light Natural Medicine Research Foundation, is known to have Thalassemia B+.