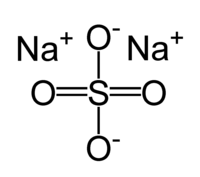
Sodium sulfate
2007 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Sodium sulfate | |
|---|---|
 |
|
| General | |
| Systematic name | Sodium sulfate |
| Other names | Sodium sulphate Salt cake Thenardite (mineral) Glauber's salt (decahydrate) Sal mirabilis (decahydrate) Mirabilite (decahydrate) Trona |
| Molecular formula | Na2SO4 |
| Hydrates | Heptahydrate: Na2SO4·7H2O Decahydrate: Na2SO4·10H2O |
| Molar mass | 142.04214 g/mol ( anhydrous) 268.14924 g/mol (heptahydrate 322.19514 g/mol (decahydrate) |
| Appearance | White crystalline solid, hygroscopic |
| CAS number | [7757-82-6] (anhydrous) [7727-73-3] (decahydrate) |
| Properties | |
| Density | 2.68 g/cm3, anhydrous (orthorhombic form) 1.464 g/cm3, decahydrate |
| Solubility in water | 4.76 g/100 ml (0 °C) 42.7 g/100 ml (100 °C) |
| In ethanol | insoluble |
| Melting point | 884 °C (1157 K) anhydrous 32.4 °C decahydrate |
| Structure | |
| Coordination geometry |
? |
| Crystal structure | monoclinic, orthorhombic or hexagonal |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Irritant |
| R/S statement | None |
| RTECS number | WE1650000 (anhydrous) |
| NFPA 704 | |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Sodium hydrogen sulfate Sodium sulfite Sodium bisulfite Sodium persulfate |
| Other cations | Lithium sulfate Potassium sulfate Magnesium sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references |
|
Sodium sulfate is an important compound of sodium. When anhydrous, it is a white crystalline solid of formula Na2SO4. The deca hydrate, Na2SO4•10H2O, is known as Glauber's salt. Sodium sulfate is mainly used for the manufacture of detergents and in the Kraft process of paper pulping, although it has many other uses. About half of the world's production is from the natural mineral form of the decahydrate (mirabilite), and half from by-products of chemical processes.
History
Glauber's salt, the decahydrate also known as sal mirabilis, is named after Johann Glauber, who discovered it in the 17th century. The white or colorless crystals were originally used as a laxative.
Physical and chemical properties
Na2SO4 is chemically very stable, being unreactive toward most oxidising or reducing agents at normal temperatures. At high temperatures, it can be reduced to sodium sulfide. It is a neutral salt, which forms aqueous solutions with pH of 7. The neutrality such solutions reflects the fact that Na2SO4 is derived, formally speaking, from a strong acid (sulfuric acid) and a strong base (sodium hydroxide). Sodium sulfate reacts with an equivalent amount of sulfuric acid to give an equilibrium concentration o the acid salt sodium hydrogen sulfate:
- Na2SO4( aq) + H2SO4(aq) → 2 NaHSO4(aq)
In fact the equilibrium is very complex and dependent on concentration and temperature, with other acid salts being present.
Na2SO4 is a typical ionic sulfate, containing Na+ ions and SO42− ions. Aqueous solutions can produce precipitates when combined with salts of Ba2+ or Pb2+, which form insoluble sulfates:
Na2SO4(aq) + BaCl2(aq) → 2 NaCl(aq) + BaSO4( s)
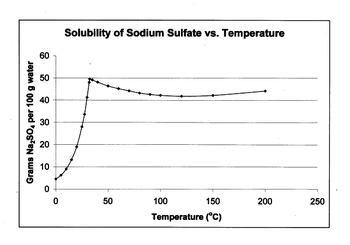
Sodium sulfate has unusual solubility characteristics in water, 3 as shown in the graph below. Its solubility rises more than tenfold between 0 °C to 32.4 °C, where it reaches a maximum of 49.7 g Na2SO4 per 100 g water. At this point the solubility curve changes slope, and the solubility becomes almost independent of temperature. In the presence of NaCl, the solubility of Na2SO4 is markedly diminished. Such changes provide the basis for the use of sodium sulfate in passive solar heating systems, as well is in the preparation and purification of sodium sulfate.
This nonconformity can be explained in terms of hydration, since 32.4 °C corresponds with the temperature at which the crystalline decahydrate (Glauber's salt) changes to give a sulfate liquid phase and an anhydrous solid phase.
Occurrence
About half of the world's production of the decahydrate (Glauber's salt) is from the natural mineral form mirabilite - found in lake beds in southern Saskatchewan, for example. In 1990, Mexico and Spain were the world's main producers of natural sodium sulfate (each around 500 000 tonnes), with USSR, USA and Canada also important (around 350 000 tonnes each).
Anhydrous sodium sulfate occurs in arid environments as the mineral thenardite, which is less common than mirabilite. It slowly turns to mirabilite in damp air.
Manufacture
About half of the world's sodium sulfate comes from natural sources (see above), while the other half is produced as a by-product of other processes. The most important of these is the production of hydrochloric acid from sodium chloride (salt) and sulfuric acid (the Mannheim process), in which case the Na2SO4 is known as salt cake:
- 2 NaCl + H2SO4 → Na2SO4 + 2 HCl
Alternatively, Na2SO4 can be produced from sulfur dioxide using the Hargreaves process:
- 4 NaCl + 2 SO2 + O2 + 2 H2O → 2 Na2SO4 + 4 HCl
In the US and UK, Na2SO4 is as a major by-product of the manufacture of sodium dichromate. Other sources of Na2SO4 include a myriad of processes where leftover sulfuric acid is neutralised by sodium hydroxide. This method is also the most convenient laboratory preparation.
- 2 NaOH( aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O( l)
Bulk sodium sulfate is usually purified via the decahydrate form, since the anhydrous form tends to attract iron compounds and organic compounds. The anhydrous form is easily produced from the hydrated form by gentle warming.
Uses
In 1995, bulk sodium sulfate sold for around $70 per tonne in the US, making it a very cheap material. Probably the largest use for sodium sulfate today is as a filler in powdered home laundry detergents. Total consumption of Na2SO4 in Europe was around 1.6 million tonnes in 2001, of which 80% was used for detergents. However this use is waning as domestic consumers are increasingly switching to liquid detergents that do not include the chemical.
Another major use for Na2SO4, particularly in the US, is in the Kraft process for the manufacture of wood pulp. Organics present in the "black liquor" from this process are burnt to produce heat, needed to drive the reduction of sodium sulfate to sodium sulfide. However, this process is being replaced to some extent by newer processes; use of Na2SO4 in the US pulp industry declined from 980 000 tonnes in 1970 to only 210 000 tonnes in 1990.
The glass industry also provides another significant application for sodium sulfate, consuming around 30 000 tonnes in the US in 1990 (4% of total US consumption). It is used as a "fining agent", to help remove small air bubbles from molten glass. It also fluxes the glass, and prevents scum formation of the glass melt during refining.
Sodium sulfate is important in the manufacture of textiles, particularly in Japan. It helps in "levelling", reducing negative charges on fibres so that dyes can penetrate evenly. Unlike the alternative sodium chloride, it does not corrode the stainless steel vessels used in dyeing.
Glauber's salt, the decahydrate, was formerly used as a laxative. It has also been proposed for heat storage in passive solar heating systems.6 This takes advantage of the unusual solubility properties (see above), and the high heat of crystallisation (78.2 kJ/mol). Other uses for sodium sulfate include frosting windows, in carpet fresheners, starch manufacture and as an additive to cattle feed. In the laboratory, anhydrous sodium sulfate is widely used as an inert drying agent for organic solutions; Na2SO4 is added to the solution until the crystals no longer clump together.
Precautions
Although sodium sulfate is generally regarded as non-toxic, handle it with care.
Suppliers/Manufacturers
- Elementis Chromium
- Meishan Dongpo District Yinfeng Chemical Co.
- Cooper
- Great LakesMinerals Co.
- Chinatrona