Chromatophore
2007 Schools Wikipedia Selection. Related subjects: General Biology
Chromatophores are pigment-containing and light-reflecting cells found in amphibians, fish, reptiles, crustaceans, and cephalopods. They are largely responsible for generating skin and eye colour in cold-blooded animals and are generated in the neural crest during embryonic development. Mature chromatophores are grouped into subclasses based on their colour (more properly " hue") under white light: xanthophores (yellow), erythrophores (red), iridophores ( reflective / iridescent), leucophores (white), melanophores (black/brown) and cyanophores (blue). The term can also refer to coloured, membrane associated vesicles found in some forms of photosynthetic bacteria.
Some species can rapidly change colour through mechanisms that translocate pigment and reorient reflective plates within chromatophores. This process, often used as a type of camouflage, is called physiological colour change. Cephalopods such as octopus have complex chromatophore organs controlled by muscles to achieve this, while vertebrates such as chameleons generate a similar effect by cell signaling. Such signals can be hormones or neurotransmitters and may be initiated by changes in mood, temperature, stress or visible changes in local environment.
Unlike cold-blooded animals, mammals and birds have only one class of chromatophore-like cell type: the melanocyte. The cold-blooded equivalent, melanophores, are studied by scientists to understand human disease and used as a tool in drug discovery.
Classification
Invertebrate pigment-bearing cells were first described as chromoforo in an Italian science journal in 1819. The term chromatophore was adopted later as the name for pigment bearing cells derived from the neural crest of cold-blooded vertebrates and cephalopods. The word itself comes from the Greek words khrōma (χρωμα) meaning "colour," and phoros (φορος) meaning "bearing". In contrast, the word chromatocyte (cyte or κυτε being Greek for "cell") was adopted for the cells responsible for colour found in birds and mammals. Only one such cell type, the melanocyte, has been identified in these animals.
It wasn't until the 1960s that the structure and colouration of chromatophores were understood well enough to allow the development of a system of sub-classification based on their appearance. This classification system persists to this day even though more recent studies have revealed that certain biochemical aspects of the pigments may be more useful to a scientific understanding of how the cells function.
The colour-related biochemicals fall into distinct classes: biochromes and schemochromes. The biochromes include true pigments, such as carotenoids and pteridines. These pigments selectively absorb parts of the visible light spectrum that makes up white light while permitting other wavelengths to reach the eye of the observer. Schemochromes have a significant effect on the perceived colours of cells although they are not actually pigments themselves. Instead, the schemochromes, though colourless, produce iridescent colours, notably silver and gold, by diffusion, interference, and scattering of light.
While all chromatophores contain pigments or reflecting structures (except when there has been a genetic mutation resulting in a disorder like albinism), not all pigment containing cells are chromatophores. Haem, for example, is a biochrome responsible for the red appearance of blood. It is primarily found in red blood cells (erythrocytes), which are generated in bone marrow throughout the life of an organism, rather than being formed during embryological development. Therefore erythrocytes are not classified as chromatophores.
Xanthophores and erythrophores
Chromatophores that contain large amounts of yellow pteridine pigments are named xanthophores and those with an excess of red/ orange carotenoids termed erythrophores. It was discovered that pteridine and carotenoid containing vesicles are sometimes found within the same cell, and that the overall colour depends on the ratio of red and yellow pigments. Therefore the distinction between these chromatophore types is essentially arbitrary. The capacity to generate pteridines from guanosine triphosphate is a feature common to most chromatophores, but xanthophores appear to have supplemental biochemical pathways that result in an excess accumulation of yellow pigment. In contrast, carotenoids are metabolised from the diet and transported to erythrophores. This was first demonstrated by rearing normally green frogs on a diet of carotene-restricted crickets. The absence of carotene in the frog's diet meant the red/orange carotenoid colour 'filter' was not present in erythrophores. This resulted in the frog appearing blue in colour, instead of green.
Iridophores and leucophores
Iridophores, sometimes also called guanophores, are pigment cells that reflect light using plates of crystalline schemochromes made from guanine. When illuminated they generate iridescent colours because of the diffraction of light within the stacked plates. Orientation of the schemochrome determines the nature of the colour observed. By using biochromes as coloured filters, iridophores create an optical effect known as Tyndall or Rayleigh scattering, producing bright blue or green colours. A related type of chromatophore, the leucophore, is found in some fish species. Like iridophores, they utilize crystalline purines to reflect light, providing the bright white colour seen in some fish. As with xanthophores and erythrophores, the distinction between iridophores and leucophores in fish is not always obvious, but generally iridophores are considered to generate iridescent or metallic colours while leucophores produce reflective white hues.
Melanophores
Melanophores contain eumelanin, a type of melanin, that appears black or dark brown because of its light absorbing qualities. It is packaged in vesicles called melanosomes and distributed throughout the cell. Eumelanin is generated from tyrosine in a series of catalysed chemical reactions. It is a complex chemical containing units of dihydroxyindole and dihydroxyindole-2- carboxylic acid with some pyrrole rings. The key enzyme in melanin synthesis is tyrosinase. When this protein is defective, no melanin can be generated resulting in certain types of albinism. In some amphibian species there are other pigments packaged alongside eumelanin. For example, a novel deep red coloured pigment was identified in the melanophores of phyllomedusine frogs. This was subsequently identified as pterorhodin, a pteridine dimer that accumulates around eumelanin. While it is likely that other lesser studied species have complex melanophore pigments, it is nevertheless true that the majority of melanophores studied to date do contain eumelanin exclusively.
Humans have only one class of pigment cell, the mammalian equivalent of melanophores, to generate skin, hair and eye colour. For this reason, and because the large number and contrasting colour of the cells usually make them very easy to visualise, melanophores are by far the most widely studied chromatophore. However, there are differences between the biology of melanophores and melanocytes. In addition to eumelanin, melanocytes can generate a yellow/red pigment called phaeomelanin.
Cyanophores
In 1995 it was demonstrated that the vibrant blue colours in some types of mandarin fish are not generated by schemochromes. Instead, a cyan biochrome of unknown chemical nature is responsible. This pigment, found within vesicles in at least two species callionymid fish, is highly unusual in the animal kingdom, as all other blue colourings thus far investigated are schemochromatic. Therefore a novel chromatophore type, the cyanophore, was proposed. Although they appear unusual in their taxonomic restriction, there may be cyanophores (as well as further unusual chromatophore types) in other fish and amphibians. For example, bright coloured chromatophores with undefined pigments have been observed in both poison dart frogs and glass frogs.
Pigment translocation
Many species have the ability to translocate the pigment inside chromatophores, resulting in an apparent change in colour. This process, known as physiological colour change, is most widely studied in melanophores, since melanin is the darkest and most visible pigment. In most species with a relatively thin dermis, the dermal melanophores tend to be flat and cover a large surface area. However, in animals with thick dermal layers, such as adult reptiles, dermal melanophores often form three-dimensional units with other chromatophores. These dermal chromatophore units (DCU) consist of an uppermost xanthophore or erythrophore layer, then an iridophore layer, and finally a basket-like melanophore layer with processes covering the iridophores.
Both types of dermal melanophores are important in physiological colour change. Flat dermal melanophores will often overlay other chromatophores so when the pigment is dispersed throughout the cell the skin appears dark. When the pigment is aggregated towards the centre of the cell, the pigments in other chromatophores are exposed to light and the skin takes on their hue. Similarly, after melanin aggregation in DCUs, the skin appears green through xanthophore (yellow) filtering of scattered light from the iridophore layer. On the dispersion of melanin, the light is no longer scattered and the skin appears dark. As the other biochromatic chomatophores are also capable of pigment translocation, animals with multiple chromatophore types can generate a spectacular array of skin colours by making good use of the divisional effect.,
The control and mechanics of rapid pigment translocation has been well studied in a number of different species, particularly amphibians and teleost fish. , It has been demonstrated that the process can be under hormonal, neuronal control or both. Neurochemicals that are known to translocate pigment include noradrenaline, through its receptor on the surface on melanophores. The primary hormones involved in regulating translocation appear to be the melanocortins, melatonin and melanin concentrating hormone (MCH), that are produced mainly in the pituitary, pineal gland and hypothalamus respectively. These hormones may also be generated in a paracrine fashion by cells in the skin. At the surface of the melanophore the hormones have been shown to activate specific G-protein coupled receptors that, in turn, transduce the signal into the cell. Melanocortins result in the dispersion of pigment, while melatonin and MCH results in aggregation.
Numerous melanocortin, MCH and melatonin receptors have been identified in fish and frogs, including a homologue of MC1R, a melanocortin receptor known to regulate skin and hair colour in humans. Inside the cell, cyclic adenosine monophosphate (cAMP) has been shown to be an important second messenger of pigment translocation. Through a mechanism not yet fully understood, cAMP influences other proteins to drive molecular motors carrying pigment containing vesicles along both microtubules and microfilaments.,,
Background adaptation
Most fish, reptiles and amphibians undergo a limited physiological colour change in response to a change in environment. This type of camouflage, known as background adaptation, most commonly appears as a slight darkening or lightening of skin tone to approximately mimic the hue of the immediate environment. It has been demonstrated that the background adaptation process is vision dependent (it appears the animal needs to be able to see the environment to adapt to it), and that melanin translocation in melanophores is the major factor in colour change. Some animals, such as chameleons and anoles, have a highly developed background adaptation response capable of generating a number of different colours very rapidly. They have adapted the capability to change colour in response to temperature, mood, stress levels and social cues, rather than to simply mimic their environment.
Development
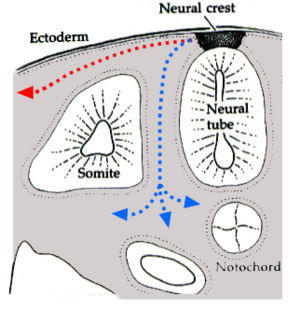
During vertebrate embryonic development, chromatophores are one of a number of cell types generated in the neural crest, a paired strip of cells arising at the margins of the neural tube. These cells have the ability to migrate long distances, allowing chromatophores to populate many organs of the body, including the skin, eye, ear and brain. Leaving the neural crest in waves, chromatophores take either a dorsolateral route through the dermis, entering the ectoderm through small holes in the basal lamina, or a ventromedial route between the somites and the neural tube. The exception to this is the melanophores of the retinal pigmented epithelium of the eye. These are not derived from the neural crest, instead an outpouching of the neural tube generates the optic cup which, in turn, forms the retina.
When and how multipotent chromatophore precursor cells (called chromatoblasts) develop into their daughter subtypes is an area of ongoing research. It is known in zebrafish embryos, for example, that by 3 days after fertilization each of the cell classes found in the adult fish — melanophores, xanthophores and iridophores — are already present. Studies using mutant fish have demonstrated that transcription factors such as kit, sox10 and mitf are important in controlling chromatophore differentiation. If these proteins are defective, chromatophores may be regionally or entirely absent, resulting in a leucistic disorder.
Practical applications
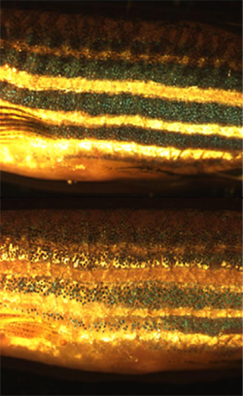
In addition to basic research into better understanding of chromatophores themselves, the cells are used for applied research purposes. For example, zebrafish larvae are used to study how chromatophores organise and communicate to accurately generate the regular horizontal striped pattern in seen in adult fish. This is seen as a useful model system for understanding patterning in the evolutionary developmental biology field. Chromatophore biology has also been used to model human condition or disease, including melanoma and albinism. Recently the gene responsible for the melanophore-specific golden zebrafish strain, Slc24a5, was shown to have a human equivalent that strongly correlates with skin colour.
Chromatophores are also used as a biomarker of blindness in cold-blooded species, as animals with certain visual defects fail to background adapt to light environments. Human homologues of receptors that mediate pigment translocation in melanophores are thought to involved in processes such as appetite suppression and tanning, making them attractive targets for drugs. Therefore pharmaceutical companies have developed a biological assay for rapidly identifying potential bioactive compounds using melanophores from the African clawed frog. Other scientists have developed techniques for using melanophores as biosensors, and for rapid disease detection (based on the discovery that pertussis toxin blocks pigment aggregation in fish melanophores). Potential military applications of chromatophore mediated colour changes have been proposed, mainly as a type of active camouflage.
Cephalopod chromatophores
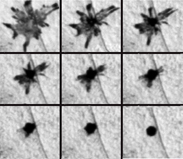
Coleoid cephalopods have complex multicellular 'organs' which they use to change colour rapidly. This is most notable in brightly coloured squid, cuttlefish and octopuses. Each chromatophore unit is composed of a single chromatophore cell and numerous muscle, nerve, glial and sheath cells. Inside the chromatophore cell, pigment granules are enclosed in an elastic sac, called the cytoelastic sacculus. To change colour the animal distorts the sacculus form or size by muscular contraction, changing its translucency, reflectivity or opacity. This differs from the mechanism used in fish, amphibians and reptiles, in that the shape of the sacculus is being changed rather than a translocation of pigment vesicles within the cell. However a similar effect is achieved.
Octopuses operate chromatophores in complex, wavelike chromatic displays, resulting in a variety of rapidly changing colour schemes. The nerves that operate the chromatophores are thought to be positioned in the brain, in a similar order to the chromatophores they each control. This means the pattern of colour change matches the pattern of neuronal activation. This may explain why, as the neurons are activated one after another, the colour change occurs in waves. Like chameleons, cephalopods use physiological colour change for social interaction. They are also among the most skilled at background adaptation, having the ability to match both the colour and the texture of their local environment with remarkable accuracy.
Bacteria
Chromatophores are also found in membranes of phototrophic bacteria. Used primarly for photosynthesis, they contain bacteriochlorophyll pigments and carotenoids. In purple bacteria, such as Rhodospirillum rubrum the light-harvesting proteins are intrinsic to the chromatophore membranes. However, in green sulphur bacteria they are arranged in specialised antenna complexes called chlorosomes.