Adenosine triphosphate
2007 Schools Wikipedia Selection. Related subjects: Chemical compounds
Adenosine 5'-triphosphate (ATP), discovered in 1929 by Karl Lohmann, is a multifunctional nucleotide primarily known in biochemistry as the " molecular currency" of intracellular energy transfer. In this role ATP transports chemical energy within cells. It is produced as an energy source during the processes of photosynthesis and cellular respiration. The structure of this molecule consists of a purine base ( adenine) attached to the 1' carbon atom of a pentose ( ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose. ATP is also one of four monomers ( nucleotides) required for the synthesis of ribonucleic acids. Furthermore, in signal transduction pathways, ATP is used to provide the phosphate for protein kinase reactions.
Chemical properties
ATP consists of adenosine - itself composed of an adenine ring and a ribose sugar - and three phosphate groups (triphosphate). The phosphoryl groups, starting with the group closest to the ribose, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. The system of ATP and water under standard conditions and concentrations is extremely rich in chemical energy; the bond between the second and third phosphate groups is loosely said to be particularly high in energy. Strictly speaking, the bond itself is not high in energy (like all chemical bonds it requires energy to break), but energy is produced when the bond is broken and water is allowed to react with the two products. Thus, energy is produced from the new bonds formed between ADP and water, and between phosphate and water.
The net change in energy at Standard Temperature and Pressure of the decomposition of ATP into hydrated ADP and hydrated inorganic phosphate is -12 kcal / mole in vivo (inside of a living cell) and -7.3 kcal / mole in vitro (in laboratory conditions). This large release in energy makes the decomposition of ATP in water extremely exergonic, and hence useful as a means for chemically storing energy. Again, the energy is actually released as hydrolysis of the phosphate-phosphate bonds is carried out.
This energy can be used by a variety of enzymes, motor proteins, and transport proteins to carry out the work of the cell. Also, the hydrolysis yields free inorganic Pi and ADP, which can be broken down further to another Pi and AMP.
ATP can also be broken down to AMP directly, with the formation of PPi. This last reaction has the advantage of being an effectively irreversible process in aqueous solution.
Ionisation of ATP in biological systems
Given the reaction:
- HATP3− ↔ H+ + ATP4−
The acidity constant is Ka = 3.16 x 10−7 giving ![\scriptstyle \frac{[\mathrm{ATP}^{4-}]}{[\mathrm{HATP}^{3-}]}=\frac{3.16 \times 10^{-7}}{[\mathrm{H}^+]}](../../images/391/39120.png) . In biological systems such as the cytosol ( pH 7.0) or the extracellular fluid (pH 7.4), ATP4− is the dominant form (76% of the total ATP for pH 7.0).
. In biological systems such as the cytosol ( pH 7.0) or the extracellular fluid (pH 7.4), ATP4− is the dominant form (76% of the total ATP for pH 7.0).
ATP synthesis
ATP can be produced by redox reactions using simple and complex sugars ( carbohydrates) or lipids as an energy source. For ATP to be synthesised from complex fuels, they first need to be broken down into their basic components. Carbohydrates are hydrolysed into simple sugars, such as glucose and fructose. Fats ( triglycerides) are metabolised to give fatty acids and glycerol.
The overall process of oxidizing glucose to carbon dioxide is known as cellular respiration and can produce up to 30 molecules of ATP from a single molecule of glucose. ATP can be produced by a number of distinct cellular processes; the three main pathways used to generate energy in eukaryotic organisms are glycolysis, the citric acid cycle/ oxidative phosphorylation, and beta-oxidation. The majority of this ATP production by a non- photosynthetic aerobic eukaryote takes place in the mitochondria, which can make up nearly 25% of the total volume of typical cell.
Glycolysis
In glycolysis, glucose and glycerol are metabolised to pyruvate in the cytosol via the glycolytic pathway. This generates a net two molecules of ATP through substrate phosphorylation catalyzed by two enzymes: PGK and pyruvate kinase. Two molecules of NADH are also produced, which can be oxidized via the electron transport chain and result in the generation of additional ATP by ATP synthase. The pyruvate generated by glycolysis can function as a substrate for the Krebs Cycle.
Citric acid cycle
In the mitochondrion, pyruvate is oxidized by pyruvate dehydrogenase to acetyl CoA, which is fully oxidized to carbon dioxide by the citric acid cycle (also known as the Krebs Cycle). Every "turn" of the citric acid cycle produces two molecules of carbon dioxide, one molecule of the ATP equivalent guanosine triphosphate (GTP) through substrate-level phosphorylation catalyzed by succinyl CoA synthetase, three molecules of the reduced coenzyme NADH, and one molecule of the reduced coenzyme FAHD2. Both of these latter molecules are recycled to their oxidized states (NAD+ and FAD, respectively) via the electron transport chain, which generates additional ATP by oxidative phosphorylation coupled to ATP synthesis. The oxidation of an NADH molecule results in the synthesis of about 3 ATP molecules, and the oxidation of one FADH2 yields about 2 ATP molecules. The majority of cellular ATP is generated by this process. Although the citric acid cycle itself does not involve molecular oxygen, it is an obligately aerobic process because O2 is needed to recycle the reduced NADH and FADH2 to their oxidized states. In the absence of oxygen the citric acid cycle will cease to function due to the lack of available NAD+ and FAD.
The generation of ATP by the mitochondrion from cytosolic NADH relies on the malate-aspartate shuttle (and to a lesser extent, the glycerol-phosphate shuttle) because the inner mitochondrial membrane is impermeable to NADH and NAD+. Instead of transferring the generated NADH, a malate dehydrogenase enzyme converts oxaloacetate to malate, which is translocated to the mitochondrial matrix. Another malate dehydrogenase-catalyzed reaction occurs in the opposite direction, producing oxaloacetate and NADH from the newly transported malate and the mitochondrion's interior store of NAD+. A transaminase converts the oxaloacetate to aspartate for transport back across the membrane and into the intermembrane space.
It is the passage of electron pairs from NADH and FADH2 through the electron transport chain that powers the pumping of protons out of the mitrochondrial matrix and into the intermembrane space, which results in a proton motive force that is the net effect of a pH gradient and an electric potential gradient across the inner mitochondrial membrane. Flow of protons down the potential gradient - that is, from the intermembrane space to the matrix - provides the driving force for ATP synthesis by the protein complex ATP synthase, which contains a unique rotor subunit that physically rotates relative to the static portions of the protein during ATP synthesis.
Most of the ATP synthesized in the mitochondria will be used for cellular processes in the cytosol; thus it must be exported from its site of synthesis in the mitochondrial matrix. The inner membrane contains antiporters that are integral membrane proteins used to exchange newly synthesized ATP in the matrix for ADP in the intermembrane space.
Beta-oxidation
Fatty acids can also be broken down to acetyl CoA by beta-oxidation of acyl CoA molecules. Each turn of this cycle reduces the length of the acyl chain by two carbon atoms and produces one NADH and one FADH2 molecule, which are used to generate ATP by oxidative phosphorylation. Because NADH and FADH2 are energy-rich molecules, dozens of ATP molecules can be generated by the beta-oxidation of a single long acyl chain.
Anaerobic respiration
Anaerobic respiration or fermentation entails the generation of energy via the process of oxidation in the absence of O2 as an electron acceptor. In most eukaryotes, glucose is used as both an energy store and an electron donor. The formula for the oxidation of glucose to lactic acid is:
- C6H12O6 ---> 2C3H6O3 + 2 ATP
ATP replenishment by nucleoside diphosphate kinases
ATP can also be synthesized through several so-called "replenishment" reactions catalyzed by the enzyme families of nucleoside diphosphate kinases (NDKs), which use other nucleoside triphosphates as a high-energy phosphate donor, and the ATP:guanido-phosphotransferase family, which uses creatine.
- ADP + GTP
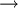 ATP + GDP
ATP + GDP
ATP production during photosynthesis
In plants, ATP is synthesized in thylakoid membrane of the chloroplast during the light-dependent reactions of photosynthesis. Some of this ATP is then used to power the Calvin cycle, which produces triose sugars.
ATP recycling
The total quantity of ATP in the human body is about 0.1 mole. The majority of ATP is not usually synthesised de novo, but is generated from ADP by the aforementioned processes. Thus, at any given time, the total amount of ATP + ADP remains fairly constant.
The energy used by human cells requires the hydrolysis of 100 to 150 moles of ATP daily which is around 50 to 75 kg. Typically, a human will use up their body weight of ATP over the course of the day. This means that each ATP molecule is recycled 1000 to 1500 times during a single day (100 / 0.1 = 1000). ATP cannot be stored, hence its consumption being followed closely by its synthesis.
Regulation of ATP production
ATP production in an aerobic eukaryotic cell is tightly regulated by allosteric mechanisms, by feedback effects, and by the substrate concentration dependence of individual enzymes within the glycolysis and oxidative phosphorylation pathways. Key control points occur in enzymatic reactions that are so energetically favorable that they are effectively irreversible under physiological conditions.
In glycolysis, hexokinase is directly inhibited by its product, glucose-6-phosphate, and pyruvate kinase is inhibited by ATP itself. The main control point for the glycolytic pathway is phosphofructokinase (PFK), which is allosterically inhibited by high concentrations of ATP and activated by high concentrations of AMP. The inhibition of PFK by ATP is unusual, since ATP is also a substrate in the reaction catalyzed by PFK; the biologically active form of the enzyme is a tetramer that exists in two possible conformations, only one of which binds the second substrate fructose-6-phosphate (F6P). The protein has two binding sites for ATP - the active site is accessible in either protein conformation, but ATP binding to the inhibitor site stabilizes the conformation that binds F6P poorly. A number of other small molecules can compensate for the ATP-induced shift in equilibrium conformation and reactivate PFK, including cyclic AMP, ammonium ions, inorganic phosphate, and fructose 1,6 and 2,6 biphosphate.
The citric acid cycle is regulated mainly by the availability of key substrates, particularly the ratio of NAD+ to NADH and the concentrations of calcium, inorganic phosphate, ATP, ADP, and AMP. Citrate - the molecule that gives its name to the cycle - is a feedback inhibitor of citrate synthase and also inhibits PFK, providing a direct link between the regulation of the citric acid cycle and glycolysis.
In oxidative phosphorylation, the key control point is the reaction catalyzed by cytochrome c oxidase, which is regulated by the availability of its substrate, the reduced form of cytochrome c. The amount of reduced cytochrome c available is directly related to the amounts of other substrates:
which directly implies this equation:
Thus, a high ratio of [NADH] to [NAD+] or a low ratio of [ADP][Pi] to [ATP] imply a high amount of reduced cytochrome c and a high level of cytochrome c oxidase activity. An additional level of regulation is introduced by the transport rates of ATP and NADH between the mitochondrial matrix and the cytoplasm.
ATP use in cells
ATP is the main energy source for the majority of cellular functions. This includes the synthesis of macromolecules, including DNA, RNA, and proteins. ATP also plays a critical role in the transport of macromolecules across cell membranes, e.g. exocytosis and endocytosis.
ATP is critically involved in maintaining cell structure by facilitating assembly and disassembly of elements of the cytoskeleton. In a related process, ATP is required for the shortening of actin and myosin filament crossbridges required for muscle contraction. This latter process is one of the main energy requirements of animals and is essential for locomotion and respiration.
Cell signaling
ATP is also a signaling molecule. ATP, ADP, or adenosine are recognised by purinergic receptors.
In humans, this signaling role is important in both the central and peripheral nervous system. Activity-dependent release of ATP from synapses, axons and glia activates purinergic membrane receptors known as P2. The P2Y receptors are metabotropic, i.e. G protein-coupled and modulate mainly intracellular calcium and sometimes cyclic AMP levels. Fifteen members of the P2Y family have been reported (P2Y1–P2Y15), although some are only related through weak homology and several (P2Y5, P2Y7, P2Y9, P2Y10) do not function as receptors that raise cytosolic calcium. The P2X ionotropic receptor subgroup comprises seven members (P2X1–P2X7) which are ligand-gated Ca2+-permeable ion channels that open when bound to an extracellular purine nucleotide. In contrast to P2 receptors (agonist order ATP > ADP > AMP > ADO), purinergic nucleotides like ATP are not strong agonists of P1 receptors which are strongly activated by adenosine and other nucleosides (ADO > AMP > ADP > ATP). P1 receptors have A1, A2a, A2b, and A3 subtypes ("A" as a remnant of old nomenclature of adenosine receptor), all of which are G protein-coupled receptors, A1 and A3 being coupled to Gi, and A3 being coupled to Gs.
Deoxyribonucleotide synthesis
In all known organisms, the deoxyribonucleotides that make up DNA are synthesized by the action of ribonucleotide reductase (RNR) enzymes on their corresponding ribonucleotides. This enzyme reduces the 2' hydroxyl group on the ribose sugar to deoxyribose, forming a deoxyribonucleotide (denoted dATP). All ribonucleotide reductase enzymes use a common sulfhydryl radical mechanism reliant on reactive cysteine residues that oxidize to form disulfide bonds in the course of the reaction. RNR enzymes are recycled by reaction with thioredoxin or glutaredoxin.
The regulation of RNR and related enzymes maintains a balance of dNTPs relative to each other and relative to NTPs in the cell. Very low dNTP concentration inhibits DNA synthesis and DNA repair and is lethal to the cell, while an abnormal ratio of dNTPs is mutagenic due to the increased likelihood of misincorporating a dNTP during DNA synthesis. Regulation of or differential specificity of RNR has been proposed as a mechanism for alterations in the relative sizes of intracellular dNTP pools under cellular stress such as hypoxia.
ATP in protein structure
Some proteins that bind ATP do so via a characteristic protein fold known as the Rossmann fold, which is a general nucleotide-binding motif that also often binds the cofactor NAD. The most common ATP-binding proteins, known as kinases, share a small number of common folds; the protein kinases, the largest kinase superfamily, all share common structural features specialized for ATP binding and phosphate transfer.
ATP in complex with proteins generally requires the presence in solution of a divalent cation, almost always magnesium, which aids in stabilizing its highly charged phosphate groups. The presence of magnesium greatly decreases the dissociation constant of ATP from its protein binding partner without affecting the ability of the kinase to catalyze its reaction once the ATP is bound. The presence of magnesium ions can serve as a mechanism for kinase regulation.
ATP analogs
Biochemistry laboratories often use in vitro studies to explore ATP-dependent molecular processes. Enzyme inhibitors of ATP-dependent enzymes such as kinases are needed to experimentally examine the binding sites and transition states involved in ATP-dependent reactions. ATP analogs are also used in X-ray crystallography to determine a protein structure in complex with ATP, often together with other substrates. Most useful ATP analogs cannot be hydrolyzed as ATP would be; instead they trap the enzyme in a structure closely related to the ATP-bound state. Adenosine 5'-(gamma-thiotriphosphate) is an extremely common ATP analog in which one of the gamma-phosphate oxygens is replaced by a sulfur atom; this molecule is hydrolyzed at a dramatically slower rate than ATP itself and functions as an inhibitor of ATP-dependent processes. In crystallographic studies, hydrolysis transition states are modeled by the bound vanadate ion. However, caution is warranted in interpreting the results of experiments using ATP analogs, since some enzymes can hydrolyze them at appreciable rates at high concentration.
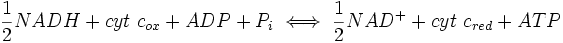
![\frac{cyt~c_{red}}{cyt~c_{ox}} = \left(\frac{[NADH]}{[NAD]^{+}}\right)^{\frac{1}{2}}\left(\frac{[ADP][P_{i}]}{[ATP]}\right)K_{eq}](../../images/391/39125.png)