Seawater
2007 Schools Wikipedia Selection. Related subjects: Geology and geophysics
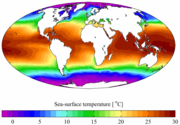
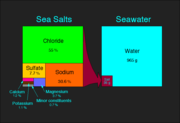
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of ~3.5%. This means that for every 1 litre (1000 mL) of seawater there are 35 grams of salts (mostly, but not entirely, sodium chloride) dissolved in it. This can be expressed as 0.6 M NaCl or 0.6 mol·L-1 (if the salinity were due entirely to NaCl, which it is not).
Although a vast majority of seawater is found in oceans with salinity around the 3.5 %, seawater is not uniformly saline throughout the world. The planet's freshest (least saline) sea water is in the eastern parts of Gulf of Finland and in the northern end of Gulf of Bothnia, both part of the Baltic Sea. The most saline open sea is the Red Sea, where high temperatures and confined circulation result in high rates of surface evaporation and there is little fresh inflow from rivers. The salinity in isolated seas and salt-water lakes (for example, the Dead Sea) can be considerably greater.
The density of seawater is between 1020 and 1030 kg·m-3. Seawater pH is limited to the range 7.5 to 8.4. The speed of sound in seawater is about 1500 m·s-1.
Geochemical explanations
| Element | Percent | Element | Percent |
|---|---|---|---|
| Oxygen | 87.7 | Sulfur | 0.0885 |
| Hydrogen | 10.8 | Calcium | 0.04 |
| Chlorine | 1.9 | Potassium | 0.0380 |
| Sodium | 1.05 | Bromine | 0.0065 |
| Magnesium | 0.1350 | Carbon | 0.0026 |
Scientific theories behind the origins of sea salt started with Sir Edmond Halley in 1715, who proposed that salt and other minerals were carried into the sea by rivers, having been leached out of the ground by rainfall runoff. Upon reaching the ocean, these salts would be retained and concentrated as the process of evaporation (see Hydrologic cycle) removed the water. Halley noted that of the small number of lakes in the world without ocean outlets (such as the Dead Sea and the Caspian Sea, see endorheic basin), most have high salt content. Halley termed this process "continental weathering".
Halley's theory is partly correct. In addition, sodium was leached out of the ocean floor when the oceans first formed. The presence of the other dominant ion of salt, chloride, results from "outgassing" of chloride (as hydrochloric acid) with other gases from Earth's interior via volcanos and hydrothermal vents. The sodium and chloride ions subsequently became the most abundant constituents of sea salt.
Ocean salinity has been stable for millions of years, most likely as a consequence of a chemical/tectonic system which recycles the salt. Since the ocean's creation, sodium is no longer leached out of the ocean floor, but instead is captured in sedimentary layers covering the bed of the ocean. One theory is that plate tectonics result in salt being forced under the continental land masses, where it is again slowly leached to the surface.
Potability
| Component | Concentration (mol . kg-1) |
|---|---|
| H2O | 53.6 |
| Cl- | 0.546 |
| Na+ | 0.469 |
| Mg2+ | 0.0528 |
| SO42- | 0.0283 |
| Ca2+ | 0.0103 |
| K+ | 0.0102 |
| CT | 0.00206 |
| Br- | 0.000844 |
| BT | 0.000416 |
| Sr2+ | 0.000091 |
| F- | 0.000068 |
Even on a ship or island in the middle of the ocean, there can be a "shortage of water" meaning, of course, a shortage of fresh water. This is described famously by a line from Samuel Taylor Coleridge's The Rime of the Ancient Mariner:
- "Water, water, every where
- Nor any drop to drink."
Seawater can be turned into drinkable ( potable) water by one of a number of desalination processes, or by diluting it with fresh water to reduce the salinity. Otherwise, it should not be drunk because of its high dissolved mineral content. In the long run, more water must be expended to eliminate these minerals (through excretion in urine) than is gained from drinking the seawater itself.
Seawater for flushing toilet
Hong Kong has an extensive use of seawater for flushing toilets citywide. More than 90% of its toilets are flushed by seawater as a means of conserving fresh water resources. The development of this approach was started in the 1960s and 1970s when water shortages became a severe problem as the population of the (then) British colony grew. This is unusual because saline water cannot be treated (in a waste water treatment plant) by the usual methods.