Nitrogen fixation
2007 Schools Wikipedia Selection. Related subjects: General Biology; General Chemistry
Nitrogen fixation is the process by which nitrogen is taken from its relatively inert molecular form (N2) in the atmosphere and converted into nitrogen compounds useful for other chemical processes (such as, notably, ammonia, nitrate and nitrogen dioxide) .
Nitrogen fixation is performed naturally by a number of different prokaryotes, including bacteria, and actinobacteria certain types of anaerobic bacteria. Microorganisms that fix nitrogen are called diazotrophs. Some higher plants, and some animals ( termites), have formed associations with diazotrophs.
Biological nitrogen fixation was discovered by the Dutch microbiologist Martinus Beijerinck.
Biological Nitrogen Fixation
Biological Nitrogen Fixation (BNF) occurs when atmospheric nitrogen is converted to ammonia by a pair of bacterial enzymes called nitrogenase . The formula for BNF is:
- N2 + 8H+ + 8e− + 16 ATP → 2NH3 + H2 + 16ADP + 16 Pi
Although ammonia (NH3) is the direct product of this reaction, it is quickly ionized to ammonium (NH4+). In free-living diazotrophs, the nitrogenase-generated ammonium is assimilated into glutamate through the glutamine synthetase/glutamate synthase pathway.
In most bacteria, the nitrogenase enzymes are very susceptible to destruction by oxygen (and many bacteria cease production of the enzyme in the presence of oxygen) . Low oxygen tension is achieved by different bacteria by: living in anaerobic conditions, respiring to draw down oxygen levels, or binding the oxygen with a protein (e.g. leghaemoglobin) . The great majority of legumes have this association, but a few genera (e.g., Styphnolobium) do not.
Non-leguminous nitrogen fixing plants
Plants from many other families have similar associations, including: * Lobaria lichen and some other lichens
- Mosquito fern (Azolla species)
- Cycads
- Gunnera
- Alder (Alnus species)
- Ceanothus (Ceanothus species)
- Wax myrtle (Myrica species)
- Mountain-mahogany (Cercocarpus species)
- Bitterbrush (Purshia tridentata)
- Buffalo berry (Shepherdia argentea)
- Ironwood (Casuarina species), Sheoak (Allocasuarina species), and other genera in the Casuarinaceae
Chemical nitrogen fixation
Nitrogen can also be artificially fixed for use in fertilizer, explosives, or in other products. The most popular method is by the Haber process. This artificial fertilizer production has achieved such scale that it is now the largest source of fixed nitrogen in the Earth's ecosystem.
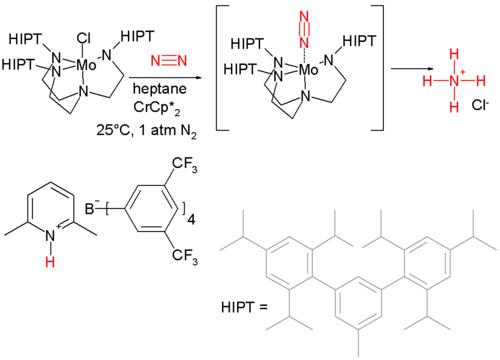
The Haber process requires high pressures and very high temperatures and active research is committed to the development of catalyst systems that convert nitrogen to ammonia at ambient temperatures. The first dinitrogen complex was discovered in 1965 based on ammonia coordinated to ruthenium ([Ru(NH3)5(N2)]2+) This discovery was followed by the first example of homolytic cleavage of nitrogen by a molybdenum complex to two equivalents of a triple bonded MoN complex (1995). The first catalytic system converting nitrogen to ammonia at room temperature and 1 atmosphere was discovered in 2003 and is based on another molybdenum catalyst, a proton source and a strong reducing agent .