Morphine
2007 Schools Wikipedia Selection. Related subjects: Chemical compounds; Health and medicine
 |
|
 |
|
|
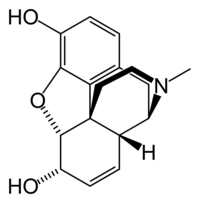
Morphine
|
|
| Systematic ( IUPAC) name | |
| 7,8-didehydro- 4,5-epoxy-17-methyl morphinan-3,6-diol |
|
| Identifiers | |
| CAS number | 57-27-2 |
| ATC code | N02 AA01 |
| PubChem | 5288826 |
| DrugBank | APRD00215 |
| Chemical data | |
| Formula | C17H19NO3 |
| Mol. weight | 285.4 |
| Pharmacokinetic data | |
| Bioavailability | ~30% |
| Protein binding | 30–40% |
| Metabolism | Hepatic 90% |
| Half life | 2–3 hours |
| Excretion | Renal 90%, biliary 10% |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
S8(AU) Schedule I(CA) Class A(UK) Schedule II(US) ℞ Prescription only |
| Dependence Liability | Extremely High |
| Routes | smoked/ inhaled, insufflated, Oral, SC, IM, IV |
Morphine ( INN) ( IPA: [ˈmɔ(ɹ)fin]) is an extremely powerful opiate analgesic drug and is the principal active agent in opium. Like other opioids, e.g. heroin, morphine acts directly on the central nervous system (CNS) to relieve pain, and at synapses of the arcuate nucleus, in particular. Side effects include impairment of mental performance, euphoria, drowsiness, lethargy, and blurred vision. It also decreases hunger, inhibits the cough reflex, and produces constipation. Morphine is highly addictive when compared to other substances, and tolerance and physical and psychological dependence develop quickly. Patients on morphine often report insomnia, visual hallucinations and nightmares. Used in ACS "Chest Pain believed to be of cardiac origin" as an adjunct to nitroglycerine providing pain relief, decreasing patient anxiety and minimal coronary artery dilation, which helps oxygenate the heart.
The word "morphine" is derived from Morpheus, the god of dreams in Greek mythology. He is the son of Somnus, god of sleep.
Medical use
Administration
Parenterally as subcutaneous, intravenous, or epidural injections. When injected, particularly intravenously, morphine produces an intense contraction sensation in the muscles and thus produces a powerful 'rush'. The military sometimes issues morphine loaded in an autoinjector.
Orally, it comes as an elixir, concentrated solution, powder (for compounding) or in tablet form. Morphine is rarely supplied in suppository form. Due to its poor oral bioavailability, oral morphine is only one-sixth to one-third of the potency of parenteral morphine. Morphine is available in extended-release capsules for chronic administration, as well as immediate-release formulations.
Uses
Morphine is used legally:
- analgesic in hospital settings for
- Pain after surgery
- Pain associated with trauma
- In the relief of severe chronic pain
- Cancer pain
- Pain from kidney stones
- Back pain
- As an adjunct to general anesthesia
- In epidural anesthesia
- For palliative care (i.e. to alleviate pain without curing the underlying reason for it)
- As an antitussive for severe cough
- As an antidiarrheal in chronic conditions (e.g., for diarrhea associated with AIDS)
Contraindications
- acute respiratory depression
- acute alcoholism
- acute pancreatitis (this may be a result of morphine use as well)
- renal failure (due to accumulation of the metabolite morphine-6-glucuronide)
Pharmacology
Indicated for:
Recreational uses:
Other uses:
|
Contraindications:
|
| Side effects: Severe:
Atypical sensations:
Cardiovascular:
Ear, nose, and throat:
Endocrinal:
Eye:
Gastrointestinal:
Hepatological:
Hematological:
Musculo skeletal:
Neurological:
Psychological:
Respiratory:
Skin:
|
Morphine is an phenanthrene opioid receptor agonist – its main effect is binding to and activating the µ-opioid receptors in the central nervous system. Activation of these receptors is associated with analgesia, sedation, euphoria, physical dependence and respiratory depression. Morphine is also a κ-opioid receptor agonist, with this action associated with spinal analgesia and miosis.
Like loperamide and other opioids, morphine acts on the myenteric plexus in the intestinal tract reduce gut motility, leading to constipation.
The effects of morphine can be countered with naloxone or naltrexone.
Morphine is primarily metabolized into morphine-3-glucuronide and morphine-6-glucuronide. Morphine-6-glucuronide (M6G) has been found to be a far more potent analgesic than morphine when dosed to rodents. M6G has also been found to be analgesic in humans. The significance of M6G formation on the observed effect of a dose of morphine is the subject of extensive debate among pharmacologists.
High doses of morphine may be fatal due to respiratory depression. Nevertheless, patients in extreme pain are able to tolerate very high doses of morphine. This is because pain stimulates respiration, thus counteracting the respiratory depression.
Chemistry
Most of the morphine produced is used to make codeine. It is also a precursor for both diamorphine and hydromorphone. Replacement of the N-methyl group of morphine with an N-phenylethyl group results in a product that is 18x morphine in it's opiate against potency.
Legal classification
- In the United Kingdom, morphine is listed as a Class A drug under the Misuse of Drugs Act 1971.
- In the United States, morphine is classified as a Schedule II drug under the Controlled Substances Act.
- In Australia, morphine is classified as a Schedule 8 drug under the Therapeutic Goods Act 1989 (2003).
- Internationally, morphine is a Schedule I drug under the Single Convention on Narcotic Drugs .
History and abuse
Morphine was first isolated in 1804 by the German pharmacist Friedrich Wilhelm Adam Sertürner (or Barnard Courtois), who named it "morphium" after Morpheus, the Greek god of dreams. But it was not until the development of the hypodermic needle ( 1853) that its use spread. It was used for pain relief, and as a "cure" for opium and alcohol addiction. Its extensive use during the American Civil War allegedly resulted in over 400,000 sufferers from the "soldier's disease" of morphine addiction, although critics believe this to be a highly erroneous and misleading claim, pointing to the glaring lack of documented post-war cases .
Heroin (diacetylmorphine) was derived from morphine in 1874. As with other drugs, its possession without a prescription was criminalized in the U.S. by the Harrison Narcotics Tax Act of 1914.
Morphine is routinely carried by soldiers on operations in an autoinjector.
Morphine was the most commonly abused narcotic analgesic in the world up until heroin was synthesized and came into use. Even today, morphine is one of the most sought after prescription narcotics by heroin addicts when heroin is scarce.
In a randomised double-blind study with crossover at an outpatient clinic in Bern, Switzerland, morphine was proven to have stronger side-effects than heroin at equianalgesic doses. Respiratory depression, miosis, sedation, itchiness, and euphoria were more pronounced with morphine.