Hydrogen peroxide
2007 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Hydrogen peroxide | |
|---|---|
 |
|
| General | |
| Systematic name | Dihydrogen dioxide |
| Other names | Hydrogen peroxide hydrogen dioxide |
| Molecular formula | H2O2 |
| Molar mass | 34.0147 g/mol. |
| Appearance | Very pale blue colour; colorless in solution. |
| CAS number | [7722-84-1] |
| Properties | |
| Density and phase | 1.4 g/cm3, liquid |
| Solubility in water | Miscible. |
| Melting point | -11 °C (262.15 K) |
| Boiling point | 150.2 °C (423.35 K) |
| Acidity (pKa) | 11.65 |
| Viscosity | 1.245 c P at 20 °C |
| Structure | |
| Molecular shape | ? |
| Dipole moment | 2.26 D |
| Hazards | |
| MSDS | 30% hydrogen peroxide msds 60% hydrogen peroxide msds |
| Main hazards | Oxidant, corrosive. |
| NFPA 704 | |
| Flash point | Non-flammable. |
| R/S statement | R: R5, R8, R20, R22,R35 S: S1, S2, S17, S26,S28, S36, S37, S39, S45 |
| RTECS number | MX0900000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | ? |
| Other cations | Sodium peroxide |
| Related compounds | Water ozone hydrazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references |
|
Hydrogen peroxide (H2O2) is a very pale blue liquid which appears colourless in a dilute solution, slightly more viscous than water. It has strong oxidizing properties and is therefore a powerful bleaching agent that has found use as a disinfectant, as an oxidizer, and in rocketry (particularly in high concentrations as high-test peroxide (HTP) as a monopropellant), and in bipropellant systems.
History
Hydrogen peroxide was first isolated in 1818 by Louis Jacques Thénard by reacting barium peroxide with nitric acid. An improved version of this process used hydrochloric acid, followed by sulfuric acid to precipitate the barium chloride byproduct. Thenard's process was used from the end of the 19th century until the middle of the 20th century. Modern manufacture methods are discussed below.
Uses
Industrial applications
About 50% of the world's production of hydrogen peroxide in 1994 was used for pulp- and paper-bleaching. Other bleaching applications are becoming more important as hydrogen peroxide is seen as an environmentally-benign alternative to chlorine-based bleaches.
Other major industrial applications for hydrogen peroxide include the manufacture of sodium percarbonate and sodium perborate, used as mild bleaches in laundry detergents. It is used in the production of certain organic peroxides such as dibenzoyl peroxide, used in polymerisations and other chemical processes. Hydrogen peroxide is also used in the production of epoxides such as propylene oxide. Reaction with carboxylic acids produces a corresponding "per-acid". Peracetic acid and meta-chloroperoxybenzoic acid (commonly abbreviated mCPBA) are prepared from acetic acid and meta-chlorobenzoic acid, respectively. The latter is commonly reacted with alkenes to give the corresponding epoxide.
Domestic uses
Diluted H2O2 (around 5%) is used to bleach human hair, hence the phrases peroxide blonde and bottle blonde. It can absorb into skin upon contact and create a local skin capillary embolism which appears as a temporary whitening of the skin. It whitens skeletons that are to be put on display. 3% H2O2 is used medically for cleaning wounds, removing dead tissue, or as an oral debriding agent. Most over-the-counter peroxide solutions are not, however, suitable for ingestion.
The Food and Drug Administration (FDA) has classified hydrogen peroxide as a Low Regulatory Priority (LRP) drug for use in controlling fungus on fish and fish eggs. See ectoparasite.
Some gardeners and hydroponics implementers have professed the value of hydrogen peroxide in their watering solutions. They claim its spontaneous decomposition releases oxygen to the plant that can enhance root development and also help treat root rot, which is cellular root death due to lack of oxygen. Laboratory tests conducted by fish culturists in recent years have demonstrated that common household hydrogen-peroxide can be used safely to provide oxygen for small fish. Citation Reference Hydrogen-peroxide releases oxygen by decomposition when it is exposed to catalysts.
Hydrogen peroxide is increasingly popular for the treatment of hydrogen sulfide and iron. Catalytic carbon and redox media perform well with hydrogen peroxide pretreatment. Generally 90% of the reaction between hydrogen peroxide and hydrogen sulfide takes place within 10 to 15 minutes, with the balance reacting in an additional 20 to 30 minutes. The sulfur in hydrogen sulfide H2S) is in the -2 state. In a neutral solution, hydrogen peroxide will oxidize hydrogen sulfide to elemental sulfur via the following reaction: 8 H2S(g) + 8 H2O2(aq) → S8(s) + 16 H2O(l)
The reaction is slow but may be catalyzed by metal ions. To be more specific for doses of chemical feed levels for oxidation of iron, manganese and hydrogen sulfide in domestic water supplies, here are some figures: Iron: For each ppm Fe feed = 0.3 - 0.5 ppm, 20 minutes Manganese: For each ppm Mn feed = 0.8 - 1.0 ppm, 20 minutes Hydrogen Sulfide: For each ppm H2S feed = 1.0 - 1.5 ppm, 30 minutes (all above figures are for minimum retention time). When more than one constituent is to be oxidized (i.e. iron & H2S) add the above values to determine the total ppm feed needed to oxidize two or more.
Hydrogen peroxide is a strong oxidizer effective in controlling sulfide and organic related odors in wastewater collection and treatment systems. It is typically applied to a wastewater system most frequently where there is a retention time of less than five hours and at least 30 minutes prior to the point where the hydrogen sulfide is released. Hydrogen peroxide will oxidize the hydrogen sulfide present and in addition promote bio-oxidation of organic odours. Hydrogen peroxide decomposes to oxygen and water adding dissolved oxygen to the system thereby reducing Biological Oxygen Demand (BOD).
Commercial peroxide, as bought at the drugstore in a 2.5%-3% solution, can be used to remove bloodstains from carpets and clothing. If a few tablespoons of peroxide are poured onto the stain, they will bubble up in the area of the blood. After a few minutes the excess liquid can be wiped up with a cloth or paper towel and the stain will be gone. Care should be taken, however, as hydrogen peroxide will bleach or discolor many fabrics.
Hydrogen peroxide is used in glow sticks as an oxidising agent. It reacts with phenyl oxalate ester to form an unstable CO2 dimer which in turn causes an added dye to reach an excited state, the latter relaxing to release photons of light.
Storage
Small quantities of many different concentrations and grades can be legally stored and used with few regulations.
Hydrogen peroxide should be stored in a container made from a material that doesn't react with the chemical. Numerous materials and processes are available, and these vary based on the concentration and grade (purity) of the hydrogen peroxide. In general, it is an oxidizer and should be stored away from fuel sources and sources of catalytic contamination. Because oxygen is formed during the natural decomposition of the peroxide, the resulting increase in pressure can cause a glass container to break. Therfore, H2O2 should be stored in vented plastic containers.
Use as propellant
H2O2 can be used either as a monopropellant (not mixed with fuel) or as the oxidizer component of a bipropellant rocket. Use as a monopropellant takes advantage of the decomposition of 70–98+% concentration hydrogen peroxide into steam and oxygen. The propellant is pumped into a reaction chamber where a catalyst (usually a silver or platinum screen) triggers decomposition, and the hot (>600 °C) oxygen/steam produced is used directly for thrust. H2O2 monopropellant produces a maximum specific impulse (Isp) of 161 s (1.6 kN·s/kg), which makes it a low-performance monopropellant. Compared to hydrazine, peroxide is less toxic, but it is also much less powerful. The famous Bell Rocket Belt used hydrogen peroxide monopropellant.
As a bipropellant, H2O2 is decomposed to burn a fuel as an oxidizer. Specific impulses as high as 350 s (3.5 kN·s/kg) can be achieved, depending on the fuel. Peroxide used as an oxidizer gives a somewhat lower Isp than liquid oxygen, but is dense, storable, noncryogenic and can be more easily used to drive gas turbines to give high pressures. It also can be used for regenerative cooling of rocket engines. Peroxide was used very successfully as an oxidizer for early World-War-II era German rockets, and for the low-cost British launchers, Black Knight and Black Arrow.
In the 1940s and 1950s, the Walter turbine used hydrogen peroxide for use in submarines while submerged; it was found to be too noisy and maintenance-demanding compared to the conventional diesel-electric power system. Some torpedoes used hydrogen peroxide as oxidizer or propellant, but this use has been discontinued by most navies for safety reasons. Hydrogen peroxide leaks were blamed for the sinkings of HMS Sidon and the Russian submarine Kursk. It was discovered, for example, by the Japanese Navy in torpedo trials, that the concentration of H2O2 in right-angle bends in HTP pipework can often lead to explosions in submarines and torpedoes.
While its application as a monopropellant for large engines has waned, small thrusters for attitude control that run on hydrogen peroxide are still in use on some satellites, and provide benefits on the spacecraft, making it easier to throttle and safer loading and handling of fuel before launch (as compared to hydrazine monopropellant). However, hydrazine is a more popular monopropellent in spacecraft because of its higher specific impulse and lower rate of decomposition.
Recently, H2O2/ propylene as an approach to inexpensive Single Stage To Orbit has been proposed; this involves a main fuel tank containing propylene, with a bladder floating in it containing the H2O2. This combination offers 15% superior ISP to O2/RP4 (a kerosene used as rocket propellant), avoiding the need for turbines, cryogenic storage or hardware, and greatly reduced cost for the construction of the booster; the potential of this and other alternate systems is discussed in some detail at Dunn Engineering which is offered as a citation.
Therapeutic use
Hydrogen peroxide has been used as an antiseptic and anti-bacterial agent for many years. While its use has decreased in recent years due to the popularity of better-smelling and more readily-available over the counter products, it is still used by many hospitals, doctors and dentists in sterilising, cleaning and treating everything from floors to Root canal procedures.
Recently, alternative medical practitioners have advocated administering doses of hydrogen peroxide intravenously in extremely low (less than one percent) concentrations for hydrogen peroxide therapy — a controversial alternative medical treatment for cancer. However, according to the American Cancer Society, "there is no scientific evidence that hydrogen peroxide is a safe, effective or useful cancer treatment." They advise cancer patients to "remain in the care of qualified doctors who use proven methods of treatment and approved clinical trials of promising new treatments." Internal use of hydrogen peroxide has a history of causing fatal blood disorders, and its recent use as a therapeutic treatment has been linked to several deaths.,
Hydrogen peroxide is GRAS (Generally Recognised As Safe) as an antimicrobial agent, an oxidizing agent and more by the US Food and Drug Administration. Hydrogen peroxide can also be used as a toothpaste when mixed with correct quantities of baking soda and salt. Like benzoyl peroxide, hydrogen peroxide is also sometimes used in the treatment of acne.
Hydrogen peroxide is also used as an emetic in veterinary practice.
Physical properties
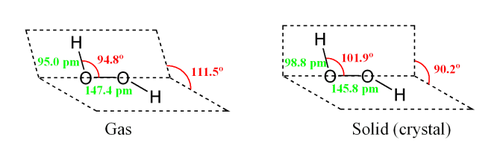
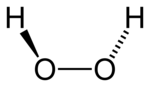
Hydrogen peroxide adopts a "skewed" shape, due to repulsion between the lone pairs on the oxygen atoms. Despite the fact that the O-O bond is a single bond, the molecule has a remarkably high barrier to complete rotation of 29.45 kJ/ mol (compared with 12.5 kJ/mol for the rotational barrier of ethane). The increased barrier is also attributed to lone-pair lone-pair repulsion. The bond angles are affected by hydrogen bonding, which is relevant to the structural difference between gaseous and crystalline forms; indeed a wide range of values is seen in crystals containing molecular H2O2.
Chemical properties
H2O2 is one of the most powerful oxidizers known -- stronger than chlorine, chlorine dioxide, and potassium permanganate. And through catalysis, H2O2 can be converted into hydroxyl radicals (.OH) with reactivity second only to fluorine.
| Oxidant | Oxidation potential, V |
|---|---|
| Fluorine | 3.0 |
| Hydroxyl radical | 2.8 |
| Ozone | 2.1 |
| Hydrogen peroxide | 1.8 |
| Potassium permanganate | 1.7 |
| Chlorine dioxide | 1.5 |
| Chlorine | 1.4 |
Hydrogen peroxide can decompose spontaneously into water and oxygen. It usually acts as an oxidizing agent, but there are many reactions where it acts as a reducing agent, releasing oxygen as a by-product. It also readily forms both inorganic and organic peroxides.
Decomposition
Hydrogen peroxide often decomposes (disproportionates) exothermically into water and oxygen gas spontaneously:
- 2 H2O2 → 2 H2O + O2 + Energy
This process is very favorable; it has a ΔHo of −98.2 kJ/ mol and a ΔGo of −119.2 kJ/mol and a ΔS of 70.5 J/mol K. The rate of decomposition is dependent on the temperature and concentration of the peroxide, as well as the pH and the presence of impurities and stabilizers. Hydrogen peroxide is incompatible with many substances that catalyse its decomposition, including most of the transition metals and their compounds. Common catalysts include manganese dioxide, potassium permanganate, and silver. The same reaction is catalysed by the enzyme catalase, found in the liver, whose main function in the body is the removal of toxic byproducts of metabolism and the reduction of oxidative stress. The decomposition occurs more rapidly in alkali, so acid is often added as a stabilizer.
Spilling high concentration peroxide on a flammable substance can cause an immediate fire fueled by the oxygen released by the decomposing hydrogen peroxide. High-strength peroxide (also called high-test peroxide, or HTP) must be stored in a vented container to prevent the buildup of oxygen gas, which would otherwise lead to the eventual rupture of the container. Any container must be made of a compatible material such as PTFE, polyethylene, stainless steel, or aluminium and undergo a cleaning process ( passivation) to remove all contamination prior to the introduction of peroxide. (Note that while compatible at room temperature, polyethylene can explode with peroxide in a fire.)
In the presence of certain catalysts, such as Fe2+ or Ti3+, the decomposition may take a different path, with free radicals such as HO· ( hydroxyl) and HOO· being formed. A combination of H2O2 and Fe2+ is known as Fenton's reagent.
Redox reactions
In aqueous solution, hydrogen peroxide can oxidize or reduce a variety of inorganic ions. When it acts as a reducing agent, oxygen gas is also produced. In acid solution Fe2+ is oxidized to Fe3+,
- 2 Fe2+(aq) + H2O2 + 2 H+(aq) → 2 Fe3+(aq) + 2H2O(l)
and sulfite (SO32−) is oxidized to sulfate (SO42−). However, potassium permanganate is reduced to Mn2+ by acidic H2O2. Under alkaline conditions, however, some of these reactions reverse; Mn2+ is oxidized to Mn4+ (as MnO2), yet Fe3+ is reduced to Fe2+.
- 2 Fe3+ + H2O2 + 2 OH− → 2 Fe2+ + 2 H2O + O2
Hydrogen peroxide is frequently used as an oxidising agent in organic chemistry. One application is for the oxidation of thioethers to sulfoxides. For example, methyl phenyl sulfide was oxidised to methyl phenyl sulfoxide in 99% yield in methanol in 18 hours (or 20 minutes using a TiCl3 catalyst):
- Ph-S-CH3 + H2O2 → Ph-S(O)-CH3 + H2O
Alkaline hydrogen peroxide is used for epoxidation of electron-deficient alkenes such as acrylic acids, and also for oxidation of alkylboranes to alcohols, the second step of hydroboration-oxidation.
Formation of peroxide compounds
Hydrogen peroxide is a weak acid, and it can form hydroperoxide or peroxide salts or derivatives of many metals. For example, with aqueous solutions of chromic acid (CrO3), it can form an unstable blue peroxide CrO(O2)2. It can also produce peroxoanions by reaction with anions; for example, reaction with borax leads to sodium perborate, a bleach used in laundry detergents:
- Na2B4O7 + 4 H2O2 + 2 NaOH → 2 Na2B2O4(OH)4 + H2O
H2O2 converts carboxylic acids (RCOOH) into peroxy acids (RCOOOH), which are themselves used as oxidizing agents. Hydrogen peroxide reacts with acetone to form acetone peroxide, and it interacts with ozone to form hydrogen trioxide. Reaction with urea produces carbamide peroxide, used for whitening teeth. An acid-base adduct with triphenylphosphine oxide is a useful "carrier" for H2O2 in some reactions.
Hydrogen peroxide reacts with ozone to form trioxidane.
Alkalinity
Hydrogen peroxide is a much weaker base than water, but it can still form adducts with very strong acids. The superacid HF/ SbF5 forms unstable compounds containing the [H3O2]+ ion.
Manufacture
Hydrogen peroxide is manufactured today almost exclusively by the autoxidation of 2-ethyl-9,10-dihydroxyanthracene to 2-ethylanthraquinone and hydrogen peroxide using oxygen from the air. The anthraquinone derivative is then extracted out and reduced back to the dihydroxy compound using hydrogen gas in the presence of a metal catalyst. The overall equation for the process is deceptively simple:
H2 + O2 → H2O2
However the economics of the process depend on effective recycling of the quinone and extraction solvents, and of the hydrogenation catalyst.
Formerly inorganic processes were used, employing the electrolysis of an aqueous solution of sulfuric acid or acidic ammonium bisulfate (NH4HSO4), followed by hydrolysis of the peroxydisulfate ((SO4)2)2− which is formed.
In 1994, world production of H2O2 was around 1.9 million tonnes, most of which was at a concentration of 70% or less. In that year bulk 30% H2O2 sold for around US $0.54 per kg, equivalent to US $1.50 per kg (US $0.68 per lb) on a "100% basis".
Concentration
Hydrogen peroxide works best as a propellant in extremely high concentrations-- roughly over 70%. Although any concentration of peroxide will generate some hot gas (oxygen plus some steam), at concentrations above approximately 67%, the heat of decomposing hydrogen peroxide becomes large enough to completely vaporize all the liquid at standard temperature. This represents a safety turning point, since decomposition of any concentration above this amount is capable of transforming the liquid entirely to heated gas (the higher the concentration, the hotter the resulting gas), and this hot steam/oxygen mixture can then be used to generate maximal thrust, power, or work.
Normal propellant grade concentrations therefore vary from 70 to 98%, with common grades of 70, 85, 90, and 98%. Many of these grades and variations are described in detail in the United States propellant specification number MIL-P-16005 Revision F, which is currently available. The available suppliers of high concentration propellant grade hydrogen peroxide are generally one of the large commercial companies which make other grades of hydrogen peroxide; including Solvay Interox, FMC, and Degussa. Other companies which have made propellant grade hydrogen peroxide in the recent past include Air Liquide and DuPont. Note that DuPont recently sold its hydrogen peroxide manufacturing business to Degussa.
Propellant grade hydrogen peroxide is available to qualified buyers. Typically this chemical is only sold to commercial companies or government institutions which have the ability to properly handle and utilize the material.
Non-professionals have purchased 70% or lower concentration hydrogen peroxide (the remaining 30% is water with traces of impurities and stabilizing materials, such as tin salts, phosphates, nitrates, and other chemical additives), and increased its concentration themselves - a potentially extremely dangerous practice that should not be encouraged. Many amateurs try distillation, but this is extremely dangerous with hydrogen peroxide; peroxide vapor can ignite or detonate depending on specific combinations of temperature and pressure. In general any boiling mass of high concentration hydrogen peroxide at ambient pressure will produce vapor phase hydrogen peroxide which can detonate. This hazard is mitigated, but not entirely eliminated with vacuum distillation. Vacuum distillation of propellant grade hydrogen peroxide is still hazardous and is best done by qualified laboratories or companies. Other approaches for concentrating hydrogen peroxide are sparging and fractional crystallization.
High concentration hydrogen peroxide is readily available in 70, 90, and 98% concentrations in sizes of 1 gallon, 30 gallon, and bulk tanker truck volumes. Propellant grade hydrogen peroxide is being used on current military systems and is in numerous defense and aerospace research and development programs. Many privately funded rocket companies are using hydrogen peroxide, notably Blue Origin. Some amateur groups have expressed interest in manufacturing their own peroxide, for their use and for sale in small quantities to others. The production of hydrogen peroxide by amateurs is potentially dangerous to both the producers of the chemical, persons in the vicinity of the chemical, and users of the chemical.
Hazards
Hydrogen peroxide, either in pure or diluted form, can pose several risks:
- Above roughly 70% concentrations, hydrogen peroxide can give off vapor that can detonate above 70 °C (158 °F) at normal atmospheric pressure. This can then BLEVE the remaining liquid. Distillation of hydrogen peroxide at normal pressures is thus highly dangerous, and must be avoided.
- Hydrogen peroxide vapors can form sensitive contact explosives with hydrocarbons such as greases. Hazardous reactions ranging from ignition to explosion have been reported with alcohols, ketones, carboxylic acids (particularly acetic acid), amines and phosphorus. The saying is 'peroxides kill chemists'.
- Hydrogen peroxide, if spilled on clothing (or other flammable materials), will preferentially evaporate water until the concentration reaches sufficient strength, then clothing will spontaneously ignite. Leather generally contains metal ions from the tanning process and will often catch fire almost immediately.
- Concentrated hydrogen peroxide (>50%) is corrosive, and even domestic-strength solutions can cause irritation to the eyes, mucous membranes and skin. Swallowing hydrogen peroxide solutions is particularly dangerous, as decomposition in the stomach releases large quantities of gas (10 times the volume of a 3% solution) leading to internal bleeding. Severe pulmonary irritation by inhalation over 10%.
Hydrogen peroxide is naturally produced as a byproduct of oxygen metabolism, and virtually all organisms possess enzymes known as peroxidases, which apparently harmlessly catalytically decomposes low concentrations of hydrogen peroxide to water and oxygen (see Decomposition above).
In one incident, several people were injured after a hydrogen peroxide spill on board Northwest Airlines Flight 957 because they mistook it for water.
For more information on the risks of working with this chemical, consult an MSDS. It reacts to make water and oxygen gas.