Helicobacter pylori
2007 Schools Wikipedia Selection. Related subjects: Organisms
| iHelicobacter pylori | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
||||||||||||||
| Scientific classification | ||||||||||||||
|
||||||||||||||
|
|
||||||||||||||
| Helicobacter pylori ((Marshall et al. 1985) Goodwin et al. 1989) |
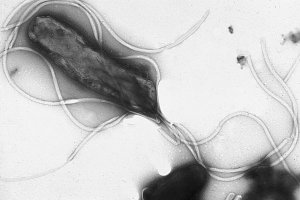
Helicobacter pylori is a bacterium that infects the mucus lining of the stomach and duodenum. Many cases of peptic ulcers, gastritis, and duodenitis are caused by H. pylori infection. However, many who are infected do not show any symptoms of disease. Helicobacter bacteria are the only known microorganisms that can thrive in the highly acidic environment of the stomach. Its helical shape (hence the name helicobacter) is thought to have evolved to penetrate and colonize the mucus lining.
History
In 1875, German scientists found spiral bacteria in the lining of the human stomach; the bacteria could not be grown in culture and the results were eventually forgotten.
In 1892, the Italian researcher Giulio Bizzozero described spiral bacteria living in the acidic environment of the stomach of dogs.
Professor Walery Jaworski of the Jagiellonian University in Kraków investigated sediments of gastric washings obtained from humans in 1899. Among some rod-like bacteria, he also found bacteria with a characteristic spiral shape, which he called Vibrio rugula. He was the first to suggest a possible role of this organism in the pathogeny of gastric diseases. This work was included in the "Handbook of Gastric Diseases" but it did not have much impact as it was written in Polish.
The bacterium was rediscovered in 1979 by Australian pathologist Robin Warren, who did further research on it with Barry Marshall beginning in 1981; they isolated the organisms from mucosal specimens from human stomachs and were the first to successfully culture them. In their original paper, Warren and Marshall contended that most stomach ulcers and gastritis were caused by colonization with this bacterium, not by stress or spicy food as had been assumed before.
The medical community was slow to recognize the role of this bacterium in stomach ulcers and gastritis, believing that no bacterium could survive for long in the acidic environment of the stomach. The community began to come around after further studies were done, including one in which Marshall drank a Petri dish of H. pylori, developed gastritis, and the bacteria were recovered from his stomach lining, thereby satisfying three out of the four Koch's postulates. Marshall's gastritis later resolved without treatment. Marshall and Warren went on to show that antibiotics are effective in the treatment of gastritis. In 1994, the National Institutes of Health (USA) published an opinion stating that most recurrent gastric ulcers were caused by H. pylori, and recommended that antibiotics be included in the treatment regimen. Evidence has been accumulating to suggest that duodenal ulcers are also associated with H. pylori infection. In 2005, Warren and Marshall were awarded the Nobel Prize in Medicine for their work on H. pylori.
Before the appreciation of the bacterium's role, stomach ulcers were typically treated with medicines that neutralize stomach acid or decrease its production. While this worked well, the ulcers very often reappeared. A traditional medication against gastritis was bismuth subsalicylate. It was often effective, but fell out of use, since its mechanism of action was a mystery. Nowadays it is quite clear that it is due to the bismuth salt acting as an antibiotic. Today, many stomach ulcers are treated with antibiotics effective against H. pylori.
The bacterium was initially named Campylobacter pyloridis, then C. pylori (after a correction to the Latin grammar) and in 1989, after DNA sequencing and other data showed that the bacterium did not belong in the Campylobacter genus, it was placed in its own genus, Helicobacter. The name pylori comes from the Greek word pylorus, which means gatekeeper, and refers to the pyloric valve (the circular opening leading from the stomach into the duodenum).
While H. pylori remains the most important known bacteria to inhabit the human stomach, several other species of the Helicobacter genus have now been identified in other mammals and some birds, and some of these can infect humans. Helicobacter species have also been found to infect the livers of certain mammals and to cause liver disease.
Structure of the bacterium
H. pylori is a spiral-shaped gram-negative bacterium, about 3 micrometres long with a diameter of about 0.5 micrometre. It has 4–6 flagella. It is microaerophilic, i.e. it requires oxygen but at lower levels than those contained in the atmosphere. It contains a hydrogenase and obtains energy by oxidizing molecular hydrogen (H2) that was produced by other intestinal bacteria. It tests positive for oxidase and catalase.
With its flagella and its helical shape, the bacterium drills into the mucus layer of the stomach, and then can be found in a number of locations: in the mucus, attached to epithelial cells, or inside vacuoles in epithelial cells. It produces adhesins which bind to membrane-associated lipids and carbohydrates and help its adhesion to epithelial cells. It excretes the enzyme urease, which converts urea into ammonia and bicarbonate. The release of ammonia is beneficial to the bacterium since it partially neutralizes the very acidic environment of the stomach (whose very purpose is to kill bacteria). Ammonia is, however, toxic to the epithelial cells, and with other products of H. pylori, including protease, catalase, and phospholipases, causes damage to those cells.
Some strains of the bacteria have a particular mechanism for "injecting" the inflammatory agent peptidoglycan from their own cell wall into epithelial stomach cells. (See below for "cagA pathogenicity island" in the section "Genome studies of different strains".) It remains unknown how this mechanism is advantageous to the bacterium.
Under conditions of environmental stress, Helicobacter will convert from the spiral to a coccoid form. This coccoid form of the organism has not been cultured, but has been found in the water supply in the US and is apparently involved in the epidemiology of the bacterium. The coccoid form has also been found to be able to adhere to gastric epithelial cells in vitro.
Infection and diagnosis
Infection may be symptomatic or asymptomatic (without visible ill effects). It is estimated that up to 70% of infection is asymptomatic.
The bacteria have been isolated from feces, saliva and dental plaque of infected patients, which suggests gastro-oral or fecal-oral as possible transmission routes.
It is estimated that about 2/3 of the world population are infected by the bacterium. Actual infection rates vary from nation to nation - the West (Western Europe, North America, Australasia) having rates around 25% and the Third World much higher. In the latter, it is common, probably due to poor sanitary conditions, to find infections in children. In the United States, infection is primarily in the older generations (about 50% for those over the age of 60 compared with 20% under 40 years) and the poorest. This is largely attributed to higher hygiene standards and widespread use of antibiotics. However, antibiotic resistance is appearing in H. pylori. There are already many metronidazole resistant strains in Europe, the United States, and developing countries.
It is widely believed that in the absence of treatment, H. pylori infection persists for life; the human immune system is not able to eradicate it. However, despite the dominance of this belief among physicians, there is actually no epidemiological evidence to support it and increasing evidence to the contrary. Because H. pylori infection is not generally detected at onset or during the acute phase, the proportion of acute infections that persist is not known, but several studies that followed the natural history in populations have reported apparent spontaneous elimination.
One can test for H. pylori infection with blood antibody or stool antigen tests, or with the carbon urea breath test (in which the patient drinks 14C- or 13C-labelled urea, which the bacterium metabolizes producing labelled carbon dioxide that can be detected in the breath), or endoscopy to provide a biopsy sample for testing for the presence of urease " rapid urease test", histology or microbial culture.
None of these test methods are completely failsafe. Blood antibody tests, for example, range from 76% to 84% sensitivity. Medication can affect H. pylori urease activity and give "false negatives" with the urea-based tests.
Treatment
In patients who are asymptomatic, treatment is not usually recommended.
In gastric ulcer patients where H. pylori is detected, normal procedure is eradication to allow the ulcer to heal. The standard first-line therapy is a one week triple-therapy. The Sydney gastroenterolgist Thomas Borody invented the first triple therapy in 1987. Today the standard triple therapy is amoxicillin, clarithromycin and a proton pump inhibitor such as omeprazole – though sometimes a different proton pump inhibitor is substituted, or metronidazole is used in place of amoxicillin in those allergic to penicillin. Such a therapy has revolutionised the treatment of gastric ulcers and has made a cure to the disease possible, where previously symptom-control using antacids, H2-antagonists or proton pump inhibitors alone was the only option.
Unfortunately, an increasing number of infected individuals are found to harbour bacteria resistant to first-line antibiotics. This results in initial treatment failure and requires additional rounds of antibiotic therapy. For resistant cases, a quadruple therapy may be used. Bismuth compounds are also effective in combination with the above drugs. For the treatment of clarithromycin- resistant strains of H. pylori the use of levofloxacin as part of the therapy has been recommended.
There is some preliminary evidence that regular consumption of broccoli sprouts might eradicate H. pylori.
Some evidence suggested that consumption of mastic gum might be able to control or even eradicate H. pylori, but later studies showed this not to be the case.
A study done on mongolian gerbils indicates that green tea extract can suppress H. pylori growth.
As explained below, some authors suggest that an H. pylori infection may be protective against certain diseases of the esophagus and cardia. Therefore, a more cautious approach than complete eradication may be necessary in some cases.
Gastric cancer connection
Gastric cancer and gastric MALT lymphoma (lymphoma of the mucosa-associated lymphoid tissue) have been associated with H. pylori, and the bacterium has been categorized as a group I carcinogen by the International Agency for Research on Cancer (IARC). While the association is reasonably strong, it is not entirely clear that there is a causal relationship involved.
Two related mechanisms by which H. pylori could promote cancer are under investigation. One mechanism involves the enhanced production of free radicals near H. pylori and an increased rate of host cell mutation. The other proposed mechanism has been called a "perigenetic pathway" and involves enhancement of the transformed host cell phenotype by means of alterations in cell proteins such as adhesion proteins. It has been proposed that H. pylori induces inflammation and locally high levels of TNF-alpha and/or interleukin 6. According to the proposed perigenetic mechanism, inflammation-associated signaling molecules such as TNF-alpha can alter gastric epithelial cell adhesion and lead to the dispersion and migration of mutated epithelial cells without the need for additional mutations in tumor suppressor genes such as genes that code for cell adhesion proteins.
Acid reflux and esophageal cancer
The infection rate with H. pylori has been decreasing in developing countries, presumably because of improved hygiene and increased use of antibiotics. Accordingly, the incidence of gastric cancer in the U.S. has fallen by 80 percent from 1900 to 2000. However, gastroesophageal reflux disease and cancer of the esophagus have increased dramatically during the same period. In 1996, Martin J. Blaser put forward the theory that H. pylori might also have a beneficial effect: by regulating the acidity of the stomach contents, it lowers the impact of regurgitation of stomach acids into the esophagus. While some favorable evidence has been accumulated, as of 2005 the theory is not universally accepted.
Genome studies of different strains
Several strains are known, and the genomes of two have been completely sequenced. The genome of the strain "26695" consists of about 1.7 million base pairs, with some 1550 genes. The two sequenced strains show large genetic differences, with up to 6% of the nucleotides differing.
Study of the H. pylori genome is centered on attempts to understand pathogenesis, the ability of this organism to cause disease. There are 62 genes in the "pathogenesis" category of the genome database. Both sequenced strains have an approximately 40 kb long Cag pathogenicity island (a common gene sequence believed responsible for pathogenesis) that contains over 40 genes. This pathogenicity island is usually absent from H. pylori strains isolated from humans who are carriers of H. pylori but remain asymptomatic.
The cagA gene codes for one of the major H. pylori virulence proteins. Bacterial strains that have the cagA gene are associated with an ability to cause severe ulcers. The cagA gene codes for a relatively long (1186 amino acid) protein. The CagA protein is transported into human cells where it may disrupt the normal functioning of the cytoskeleton. The Cag pathogenicity island has about 30 genes that code for a complex type IV secretion system. After attachment of H.pylori to stomach epithelial cells, the CagA protein is injected into the epithelial cells by the type IV secretion system. The CagA protein is phosphorylated on tyrosine residues by a host cell membrane-associated tyrosine kinase. Pathogenic strains of H. pylori have been shown to activate the epidermal growth factor receptor (EGFR), a membrane protein with a tyrosine kinase domain. Activation of the EGFR by H. pylori is associated with altered signal transduction and gene expression in host epithelial cells that may contribute to pathogenesis. It has also been suggested that a c-terminal region of the cagA protein (amino acids 873-1002) can regulate host cell gene transcription independent of protein tyrosine phosphorylation. It is thought, due to cagA's low GC content relative to the rest of the helicobacter genome, that the gene was acquired by horizontal transfer from another cagA+ bacterial species.
Each human population has a characteristic distribution of H. pylori strains that typically infect members of that population. This allows researchers to use H. pylori to study human migration patterns. It could be established that H. pylori in Amazon Indians has East Asian rather than European origins, suggesting that it arrived with the original immigrants at least 11,000 years ago.