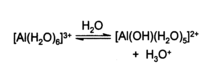Aluminium chloride
2007 Schools Wikipedia Selection. Related subjects: Chemical compounds
| Aluminium chloride | |
|---|---|
  |
|
| General | |
| Systematic name | Aluminium(III) chloride |
| Other names | Aluminium trichloride |
| Molecular formula | AlCl3 |
| Molar mass | 133.34 g mol−1 (anhydrous) 241.432 g mol−1 (hexahydrate) |
| Appearance | Pale yellow solid, hygroscopic. |
| CAS number | [7446-70-0] (anhydrous) [10124-27-3] (hexahydrate) |
| Properties | |
| Density (solid) | 2.44 g cm−3 (anhydrous) 2.40 g cm³ (hexahydrate) |
| Solubility in water | 43.9 g/100 ml (0°C) 44.9 g/100 ml (10°C) 45.8 g/100 ml (20°C) 46.6 g/100 ml (30°C) 47.3 g/100 ml (40°C) 48.1 g/100 ml (60°C) 48.6 g/100 ml (80°C) 49 g/100 ml (100°C) |
| In ethanol In chloroform In diethyl ether In CCl4 |
100 g/100 ml (12.5°C) 0.072 g/100 ml (20°C) Soluble Soluble |
| Melting point | 190 ° C (463 K) under 2.5 atm pressure |
| Boiling point | 178 ° C (351 K) ( subl) |
| Acidity (pKa) | ? |
| Structure | |
| Molecular shape | Trigonal planar ( monomeric vapour) |
| Coordination geometry | Octahedral (solid) Tetrahedral (liquid) |
| Crystal structure | 6-coordinate layer lattice |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Corrosive (C) |
| NFPA 704 | anhydrous |
| R-phrases | R34 |
| S-phrases | S1/2, S7/8, S28, S45 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Aluminium fluoride Aluminium bromide Aluminium iodide |
| Other cations | Boron trichloride Gallium(III) chloride Indium(III) chloride Thallium(III) chloride Magnesium chloride |
| Related Lewis acids | Iron(III) chloride Boron trifluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references |
|
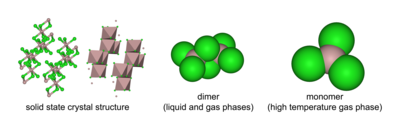
Aluminium chloride (AlCl3) is a compound of aluminium and chlorine. The solid has a low melting and boiling point, and is covalently bonded. It sublimes at 178 ° C. Molten AlCl3 conducts electricity poorly, unlike more ionic halides such as sodium chloride. It exists in the solid state as a six-coordinate layer lattice.
AlCl3 adopts the "YCl3" structure, featuring Al3+ cubic close packed layered structure. In contrast, AlBr3 has a more molecular structure, with the Al3+ centers occupying adjacent tetrahedral holes of the close-packed framework of Br− ions. Upon melting AlCl3 gives the dimer Al2Cl6, which can vaporise. At higher temperatures this Al2Cl6 dimer dissociates into trigonal planar AlCl3, which is structurally analogous to BF3.
Aluminium chloride is highly deliquescent, and it can explode in contact with water because of the high heat of hydration. It partially hydrolyses with H2O, forming some hydrogen chloride and/or hydrochloric acid. Aqueous solutions of AlCl3 are fully ionized, and thus conduct electricity well. Such solutions are found to be acidic, indicating that partial hydrolysis of the Al3+ ion is occurring. This can be described (simplified) as:
AlCl3 is probably the most commonly used non-Bronsted Lewis acid and also one of the most powerful. It finds widespread application in the chemical industry as a catalyst for Friedel-Crafts reactions, both acylations and alkylations. It also finds use in polymerization and isomerization reactions of hydrocarbons. Aluminium chloride, like similar compounds such as Aluminium chlorohydrate, is also commonly used as an antiperspirant.
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.
Chemical Properties
Aluminium chloride is a powerful Lewis acid, capable of forming stable Lewis acid-base adducts with even weak Lewis bases such as benzophenone or mesitylene. Not surprisingly it forms AlCl4− in the presence of chloride ion.
In water, partial hydrolysis forms HCl gas or H3O+, as described in the overview above. Aqueous solutions behave similarly to other aluminium salts containing hydrated Al3+ ions - for example giving a gelatinous precipitate of aluminium hydroxide upon reaction with the correct quantity of aqueous sodium hydroxide:
AlCl3( aq) + 3NaOH( aq) → Al(OH)3( s) + 3 NaCl( aq)
Preparation
Aluminium chloride is manufactured by the exothermic reaction of the elements, aluminium and chlorine. It is commercially available in large quantities.
Uses
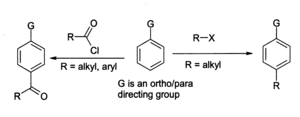
The Friedel-Crafts reaction is the major use for aluminium chloride, for example in the preparation of anthraquinone (for the dyestuffs industry) from benzene and phosgene. In the general Friedel-Crafts reaction an acyl chloride or alkyl halide reacts with an aromatic system as shown:
With benzene derivatives, the major product is the para isomer. The alkylation reaction has many associated problems, such as in Friedel-Crafts, so it is less widely used than the acylation reaction. For both reactions the aluminium chloride (and other materials and the equipment) must be moderately dry, although a trace of moisture is necessary for the reaction to proceed. A general problem with the Friedel-Crafts reaction is that the aluminium chloride " catalyst" needs to be present in full stoichiometric quantities in order for the reaction to go to completion, because it complexes strongly with the products (see chemical properties above). This makes it very difficult to recycle, so it must be destroyed after use, generating a large amount of corrosive waste. For this reason chemists are examining the use of more environmentally benign catalysts such as ytterbium(III) triflate or dysprosium(III) triflate, which can be recycled.
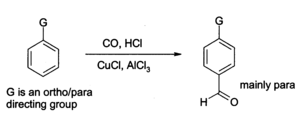
Aluminium chloride can also be used to introduce aldehyde groups onto aromatic rings, for example via the Gatterman-Koch reaction which uses carbon monoxide, hydrogen chloride and a copper(I) chloride co- catalyst):
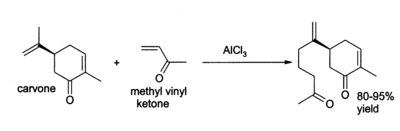
Aluminium chloride finds a wide variety of other applications in organic chemistry. For example, it can catalyse the " ene reaction", such as the addition of 3-buten-2-one (methyl vinyl ketone) to carvone:
AlCl3 is also widely used for polymerization and isomerization reactions of hydrocarbons. Important examples include the manufacture of ethylbenzene, which used to make styrene and thus polystyrene, and also production of dodecylbenzene, which is used for making detergents.
Aluminum chloride combined with aluminium in the presence of an arene can be used to synthesize bis(arene) metal complexes, e.g. bis(benzene)chromium, from certain metal halides via the so-called Fischer-Hafner synthesis.
Precautions
Avoid bringing anhydrous AlCl3 in contact with water or bases, or an explosive reaction may result. Gloves and safety goggles should be worn, along with a face shield for larger amounts. The material should be handled in a fume cupboard or chemical hood. When handled in moist air, AlCl3 rapidly absorbs moisture to become a highly acidic and sticky "goo", and it rapidly attacks many materials such as stainless steel and rubber.