Tay-Sachs disease
2007 Schools Wikipedia Selection. Related subjects: Health and medicine
| ICD- 10 | E 75.0 |
|---|---|
| ICD- 9 | 330.1 |
| OMIM | 272800 272750 |
| MedlinePlus | 001417 |
| eMedicine | ped/3016 |
Tay-Sachs disease (abbreviated TSD, also known as "GM2 gangliosidosis") is a genetic disorder, fatal in its most common variant known as Infantile Tay-Sachs disease. TSD is inherited in an autosomal recessive pattern. The disease occurs when harmful quantities of a fatty acid derivative called a ganglioside accumulate in the nerve cells of the brain. Gangliosides are present in lipids, which are components of cellular membranes, and the ganglioside GM2, implicated in Tay-Sachs disease, is especially common in the nervous tissue of the brain.
The disease is named after the British ophthalmologist Warren Tay who first described the red spot on the retina of the eye in 1881, and the American neurologist Bernard Sachs who described the cellular changes of Tay-Sachs and noted an increased prevalence in the Eastern European Jewish ( Ashkenazi) population in 1887. It has been suggested that asymptomatic carriers of Tay-Sachs (those with one defective version of HEXA and one normal gene) may have a selective advantage, but this has never been proven.
Research in the late 20th century demonstrated that Tay-Sachs disease is caused by mutations on the HEXA gene on chromosome 15. A large number of HEXA mutations have been discovered, and new ones are still being reported. These mutations reach significant frequencies in several populations. French Canadians of southeastern Quebec and Cajuns of southern Louisiana have a carrier frequency similar to Ashkenazi Jews, but they carry a different mutation. Most HEXA mutations are rare, and do not occur in genetically isolated populations. The disease can potentially occur from the inheritance of two unrelated mutations in the HEXA gene, one from each parent.
Symptoms
Tay-Sachs is classified in variant forms, based on the time of onset of neurological symptoms. The variant forms reflect diversity in the mutation base. All patients with Tay-Sachs have a "cherry-red" spot in the back of their eyes (the retina). This red spot is the area of the retina which is accentuated because of the gangliosides in the surrounding retinal ganglion cells (which are neurons of the central nervous system); the choroidal circulation is showing through "red" in this region of the fovea where where all of the retinal ganglion cells are normally pushed aside to increase visual acuity; thus the cherry-red spot is the only normal part of the retina seen. Microscopic analysis of neurons shows that they are distended from excess storage of gangliosides.
- Infantile TSD. Infants with Tay-Sachs disease appear to develop normally for the first six months of life. Then, as nerve cells become distended with gangliosides, a relentless deterioration of mental and physical abilities occurs. The child becomes blind, deaf, and unable to swallow. Muscles begin to atrophy and paralysis sets in. Death usually occurs before the age of 3.
- Juvenile TSD. Extremely rare, Juvenile Tay-Sachs disease usually presents itself in children between 2 and 10 years of age. They develop cognitive, motor, speech, and swallowing difficulties; unsteadiness of gait ( ataxia), and spasticity. Patients with Juvenile TSD usually die between 5-15 years.
- Adult/Late Onset TSD. A rare form of the disorder, known as Adult Onset Tay-Sachs disease or Late Onset Tay-Sachs disease (LOTS), occurs in patients in their 20s and early 30s. LOTS is frequently misdiagnosed, and is usually non-fatal. It is characterized by unsteadiness of gait and progressive neurological deterioration. Symptoms of LOTS, which present in adolescence or early adulthood, include speech difficulties (dysarthria), swallowing difficulties (dysphagia), unsteadiness of gait (ataxia), spasticity, cognitive decline, and psychiatric illness, particularly schizophrenic-like psychosis. Patients with LOTS frequently become wheelchair-bound in adulthood, but many live full adult lives if psychiatric and physical difficulties are accommodated. Psychiatric symptoms and seizures can be controlled with medications.
Etiology and pathogenesis
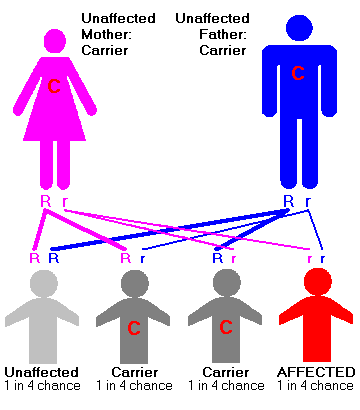
The condition is caused by insufficient activity of an enzyme called hexosaminidase A that catalyzes the biodegradation of fatty acid derivatives known as gangliosides. Gangliosides are made and biodegraded rapidly in early life as the brain develops. Patients and carriers of Tay-Sachs disease can be identified by a simple blood test that measures hexosaminidase A activity. TSD is a recessive genetic disorder, meaning that both parents must be carriers in order to give birth to an affected child. Even then, there is only a 25% chance with each pregnancy of having a child with TSD. Prenatal monitoring of pregnancies is available.
Hydrolysis of GM2-ganglioside requires three proteins. Two of them are subunits of hexosaminidase A, and the third is a small glycolipid transport protein, the GM2 activator protein (GM2A), which acts as a substrate specific cofactor for the enzyme. Deficiency in any one of these proteins leads to storage of the ganglioside, primarily in the lysosomes of neuronal cells. Tay-Sachs disease (along with GM2-gangliosidosis and Sandhoff disease) occurs because a genetic mutation inherited from both parents inactivates or inhibits this process. Most Tay-Sachs mutations appear not to affect functional elements of the protein. Instead, they cause incorrect folding or assembly of the enzyme, so that intracellular transport is disabled.
The disease results from mutations on chromosome 15 in the HEXA gene encoding the alpha-subunit of the lysosomal enzyme beta-N-acetylhexosaminidase A. More than 90 mutations have been identified to date in the HEXA gene, and new mutations are still being reported. These mutations have included base pair insertions and deletions, splice site mutations, point mutations, and other more complex patterns. Each of these mutations alter the protein product, and thus inhibit the function of the enzyme in some manner. In recent years, population studies and pedigree analysis have shown how such mutations arise and spread within small founder populations.
For example, a four base pair insertion in exon 11 (1278insTATC) results in an altered reading frame for the HEXA gene. This mutation is the most prevalent mutation in the Ashkenazi Jewish population, and leads to the infantile form of Tay-Sachs disease. The same mutation occurs in the Cajun population of southern Louisana, an American ethnic group that has been isolated for several hundred years because of linguistic differences. Researchers have speculated that it may have entered this population because a Jewish merchant family assimilated into Cajun society.
An unrelated mutation, a long sequence deletion, occurs with similar frequency in families with French Canadian ancestry, and has the same pathological effects. Like the Ashkenazi Jewish population, the French Canadian population grew rapidly from a small founder group, and remained isolated from surrounding populations because of geographic, cultural, and language barriers. In the early days of Tay-Sachs research, the mutations in these two populations were believed to be identical. Some researchers claimed that a prolific Jewish ancestor must have introduced the mutation into the French Canadian population. This theory became known as the "Jewish Fur Trader Hypothesis" among researchers in population genetics. However, subsequent research has demonstrated that the two mutations are unrelated, and pedigree analysis has traced the French Canadian mutation to a founding family that lived in southern Quebec in the late 17th century.
Tay-Sachs Disease can potentially result from the inheritance of two unrelated mutations in the HEXA gene, one from each parent. Classic infantile TSD results when a child has inherited mutations from both parents that completely inactivate the biodegradation of gangliosides. Late onset forms of the disease occur because of the diverse mutation base. Patients may technically be heterozygotes, but with two different HEXA mutations that both inactivate, alter, or inhibit enzyme activity in some way. When a patient has at least one copy of the HEXA gene that still enables some hexosaminidase A activity, a later onset form of the disease occurs.
Testing and prevention
Screening for Tay-Sachs disease was one of the first great successes of the emerging field of genetic counseling and diagnosis. Jewish communities, both inside and outside of Israel, embraced the cause of genetic screening from the 1970s on. Success with Tay-Sachs disease lead Israel to become the first country to offer free genetic screening and counseling for all couples. Israel has become a leading centre for research on genetic disease. Both the Jewish and Arab/Palestinian populations in Israel contain many ethnic and religious minority groups, and Israel's initial success with Tay-Sachs disease has lead to the development of screening programs for other diseases.
Genetic screening for carriers of Tay-Sachs disease is possible because an inexpensive enzyme assay test is available. It detects lower levels of the enzyme hexosaminidase A in serum. Developed during the 1970s, the enzyme assay test is not as accurate as genetic testing based on polymerase chain reaction (PCR) techniques, however it is cost effective for much broader use and allows screening for a disease that is rare in most populations. PCR testing is more effective when the ancestry of both parents is known, allowing for proper selection of genetic markers. Genetic counselors, working with couples that plan to conceive a child, assess risk factors based on ancestry to determine which testing methods are appropriate.
Proactive testing has been quite effective in eliminating Tays-Sachs occurrence amongst Ashkenazi Jews. Of the 10 babies born with Tay-Sachs in North America in 2003, none were born to Jewish families. In Israel, only one child was born with Tay-Sachs in 2003, and preliminary results from early 2005 indicated that none were born with the disease in 2004. Three approaches have been used to prevent or reduce the incidence of Tay-Sachs disease in the Ashkenazi Jewish population:
- Prenatal diagnosis and selective abortion. If both parents are identified as carriers, prenatal genetic testing can determine whether the fetus has inherited a defective copy of the gene from both parents. For couples who are willing to terminate the pregnancy, this eliminates the risk of Tay-Sachs, but selective abortion raises ethical issues for many families.
- Mate selection. In Orthodox Jewish circles, the organization Dor Yeshorim carries out an anonymous screening program so that couples who are likely to conceive a child with Tay-Sachs or another genetic disorder can avoid marriage. Nomi Stone of Dartmouth College describes this approach. "Orthodox Jewish high school students are given blood tests to determine if they have the Tay-Sachs gene. Instead of receiving direct results as to their carrier status, each person is given a six-digit identification number. Couples can call a hotline, if both are carriers, they will be deemed 'incompatible.' Individuals are not told they are carriers directly to avoid any possibility of stigmatization or discrimination. If the information were released, carriers could potentially become unmarriageable within the community." Anonymous testing eliminates the stigma of carriership while decreasing the rate of homozygosity in this population. Stone notes that this approach, while effective within a confined population such as Chassidic or Orthodox Jews, may not be effective in the general population.
- Preimplantation genetic diagnosis. By retrieving the mother's eggs for in vitro fertilization and conceiving a child outside the womb, it is possible to test the embryo prior to implantation. Only healthy embryos are selected for transfer into the mother's womb. In addition to Tay-Sachs disease, PGD has been used to prevent cystic fibrosis, sickle cell anaemia, Huntington disease, and other genetic disorders. However this method is expensive. It requires invasive medical technologies, and is beyond the financial means of many couples.
Therapy
There is currently no cure or treatment for TSD. Even with the best care, children with Infantile TSD die by the age of 4, and the progress of Late-Onset TSD can only be slowed, not treated.