Phenylketonuria
2007 Schools Wikipedia Selection. Related subjects: Health and medicine
| Phenylalanine | ||
| ICD- 10 | E 70.0 | |
| ICD- 9 | 270.1 | |
| OMIM | 261600 | |
| DiseasesDB | 9987 | |
| MedlinePlus | 001166 | |
| eMedicine | ped/1787 derm/712 | |
| MeSH | C10.228.140.163.100.687 | |
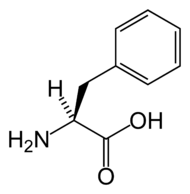
Phenylketonuria (PKU; IPA: UK /ˌfiːnʌɪlˌkiːtɘˈnjʊɘrɪɘ/ or /ˌfɛnʌɪlˌkiːtɘˈnjʊɘrɪɘ/, US /ˌfɛnɘlˌkitnˈjʊrɪɘ/ or /ˌfinɘlˌkitnˈjʊrɪɘ/) is a human genetic disorder, in which the body lacks phenylalanine hydroxylase, the enzyme necessary to metabolize phenylalanine to tyrosine. Left untreated, the disorder can cause brain damage and progressive mental retardation as a result of the accumulation of phenylalanine and its breakdown products. The incidence of occurrence of PKU is about 1 in 15,000 births, but the incidence varies widely in different human populations from 1 in 4,500 births among the population of Ireland to fewer than one in 100,000 births among the population of Finland. Phenylketonuria can also exist in mice, which have been extensively used in experiments into the correct treatment of PKU.
History
Phenylketonuria was discovered by the Norwegian physician Ivar Asbjørn Følling in 1934 when he noticed that hyperphenylalaninemia (HPA) was associated with mental retardation. In Norway, this disorder is known as Følling's disease, named after its discoverer. Dr. Følling was one of the first physicians to apply detailed chemical analysis to the study of disease. His careful analysis of the urine of two retarded siblings led him to request many physicians near Oslo to test the urine of other retarded patients. This led to the discovery of the same substance that he had found in eight other patients. The substance found was subjected to much more basic and rudimentary chemical analysis than is available today. He conducted tests and found reactions that gave rise to benzaldehyde and benzoic acid, which led him to conclude the compound contained a benzene ring. Further testing showed the melting point to be the same as phenylpyruvic acid, which indicated that the substance was in the urine. His careful science inspired many to pursue similar meticulous and painstaking research with other disorders.
Defects
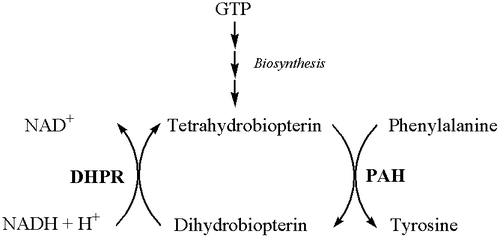
Classical PKU is caused by a defective gene for the enzyme phenylalanine hydroxylase (PAH). A rarer form of the disease occurs when PAH is normal but there is a defect in the biosynthesis or recycling of the cofactor tetrahydrobiopterin (BH4) by the patient.
This enzyme normally converts the amino acid phenylalanine to tyrosine. If, due to a faulty or missing enzyme, this reaction does not take place, levels of phenylalanine in the body can be far higher than normal, and levels of tyrosine lower than normal.
Large neutral amino acid transporter
Large neutral amino acids (LNAAs), , compete for transport across the blood brain barrier (BBB). Excessive phenylalanine in the blood saturates the large neutral amino acid transporter (LNAAT), which carries LNAAs across the BBB. Thus, excessive levels of phenylalanine significantly decrease the levels of other LNAAs in the brain. These amino acids are required for protein and neurotransmitter synthesis. Reduced protein and neurotransmitter synthesis disrupts brain development in children, leading to mental retardation.
Low levels of tyrosine also leads to lowered production of the pigment melanin, so children with this condition tend to have fairer hair and greener eyes than other members of their family. The excess phenylalanine is converted instead into the phenylketones which are excreted in the urine - hence the name for this condition. The phenylketones produced in phenylketonuria are phenylacetate, phenyllactate, phenylpyruvate, and phenylethylamine. The sweat and urine of an affected child not on the low-protein diet has a musty odour due to the phenylacetate.
Clinical Features
Untreated children with classic phenylketonuria are normal at birth, but fail to attain early developmental milestones, develop microcephaly, and demonstrate progressive impairment of cerebral function. Hyperactivity, seizures, and severe mental retardation are major clinical problems later in life. Electroencephalographic abnormalities; “mousy” odour of skin, hair, and urine (due to phenylacetate accumulation);and a tendency to hypopigmentation and eczema complete the devastating clinical picture. In contrast, affected children who are detected and treated at birth are less likely to develop neurological problems and have seizures and mental retardation, though there is a chance that it can happen.
Diagnosis
The problem is readily detectable within days of birth from a small blood sample -- the Guthrie heel prick test, so screening for phenylketonuria is done routinely in most industrialized countries, usually combined with testing thyroid function and other genetic disorders of metabolism.
In some areas, a repeat test is performed at the age of two weeks, but, if a child has been tested shortly after birth (once feeding has commenced), there is no evidence that this second test is really necessary.
Therapy
If the condition is diagnosed early enough, an affected newborn can grow up with normal brain development, but only by eating a special diet low in phenylalanine for the rest of their life. This requires severely restricting or eliminating foods high in phenylalanine, such as breast milk, meat, chicken, fish, nuts, cheese and other dairy products. Starchy foods such as potatoes, bread, pasta, and corn must also be avoided. Many diet foods and diet soft drinks that contain the sweetener aspartame must also be avoided, as aspartame is metabolized into several constituent chemicals, including phenylalanine. Supplementary formulae are used in these patients to provide the other amino acids and other necessary nutrients that would otherwise be lacking in a protein free diet. In those patients with a deficit in BH4 production or PAH has a low affinity for BH4, treatment consists of giving this cofactor as a supplement; this is referred to as BH4 responsive PKU. There are a number of potential other therapies currently under investigation, including gene therapy, and an injectable form of PAH. However, it is likely that it will be many years before these are available for use in affected individuals.
Maternal phenylketonuria
For women with PKU it is essential for the health of their child to maintain low phenylalanine levels before and during pregnancy. Though the developing fetus may only be a carrier of the PKU gene, the intrauterine environment can have very high levels of phenylalanine, which can cross the placenta. The result is the child may develop congenital heart disease, growth retardation, microcephaly and mental retardation. PKU women themselves are not at risk from additional complications during pregnancy directly as a result of their disorder. In most countries, women with PKU who wish to have children are advised to lower their blood phenylalanine levels before they become pregnant and carefully control their phenylalanine levels throughout the pregnancy. This is achieved by performing regular blood tests and adhering very strictly to a diet, generally monitored on day-to-day basis by a specialist metabolic dietitian. When low phenylalanine levels are maintained for the duration of pregnancy there are no elevated levels of risk of birth defects compared with a baby born to a non-PKU mother.
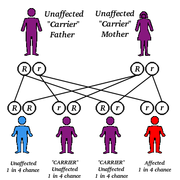
Inheritance
As PKU is an autosomal recessive genetic disorder each parent must have at least one defective allele of the gene for PAH, and the child must inherit a defective allele from each parent. As such, it is possible for a parent with a PKU phenotype to have a child without PKU if the other parent posseses at least one functional allele of the gene for PAH. A child of two parents with the PKU phenotype will always receive two defective alleles so will always have PKU. The gene for PAH is located on chromosome 12, at location 12q22-q24.2.