Duchenne muscular dystrophy
2007 Schools Wikipedia Selection. Related subjects: Health and medicine
Duchenne muscular dystrophy (DMD) (also known as muscular dystrophy - Duchenne type) is an inherited disorder characterized by rapidly progressive muscle weakness which starts in the legs and pelvis and later affects the whole body. Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy. It usually affects only males, but in rare cases it can also affect females. It is an X-linked recessive inherited disease. A milder form of this disease is known as Becker's muscular dystrophy (BMD). In Becker muscular dystrophy, most of the symptoms are similar to Duchenne, but the onset is later and the course is milder.
DMD is named after the French neurologist Guillaume Benjamin Amand Duchenne (1806-1875), who first described the disease in the 1860s. It is due to mutations in the dystrophin gene, which encodes a cell membrane protein in myocytes (muscle cells). One third of the cases are known to be caused by development of spontaneous mutations in the gene, while the remainder are inherited. Boys with DMD develop weak muscles because the muscle fibers that were present at birth are destroyed. Symptoms result in death by age 30 and respiratory failure usually results in a life expectancy of 20 years. A 1996 study found that early detection of the disease does not improve life-expectancy, and the most common cause of death is respiratory failure.
Genetics
Duchenne dystrophy is a type of dystrophinopathy which includes a spectrum of muscle disease caused by mutations in the DMD gene, which encodes the protein dystrophin. Becker's muscular dystrophy is a milder type of dystrophinopathy. Although it is caused by a defective gene, it often occurs in people from families without a known family history of the condition.
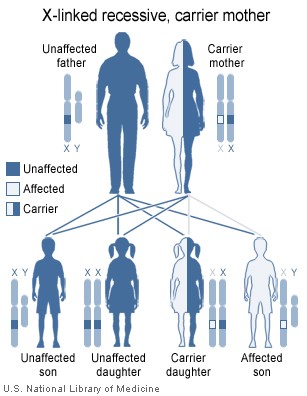
Duchenne muscular dystrophy is inherited in an X-linked recessive pattern. Because of random X inactivation, some female carriers can actually be partially affected by this disease, despite its recessive nature. X inactivation leads to women being in a state of X0, not XX as is usually thought (see below). Women who carry the defective gene can pass an abnormal X on to their sons. Since boys have an X from their mother and a Y from father, there is no second X to make up for the defective gene from the carrier mother. The sons of carrier females each have a 50% chance of having the disease, and the daughters each have a 50% chance of being carriers. Daughters of men with Duchenne will always be carriers, since they will inherit an affected X chromosome from their father (note that the diagram only shows the results from an unnaffected father) but since affected males are infertile they do not have children. Also, affected males 2/3 of the time inherited the mutation from the mother while 1/3 of the time the mutation is de novo, or new. Some females will also have very mild degrees of muscular dystrophy, and this is known as being a manifesting carrier.
Prenatal testing, such as amniocentesis, for pregnancies at risk is possible if the DMD disease-causing mutation has been identified in a family member or if informative linked markers have been identified.
In 1/3 of the cases, the disease is a result of a spontaneous or new mutation .
In some female cases, DMD is caused by skewed x inactivation. In these cases, two copies of the x chromosome exist, but for reasons currently unknown, the flawed x chromosome manifests instead of the unflawed copy. In these cases, a mosaic form of DMD is seen, in which some muscle cells are completely normal while others exhibit classic DMD findings. The effects of a mosaic form of DMD on long-term outlook is not known.
Patho-mechanism
Duchenne muscular dystrophy is caused by a mutation of the dystrophin gene whose protein product is responsible for linking the actin filaments of muscle fibres to the extracellular matrix through a protein complex containing many subunits. As a result, the sarcolemma is damaged through shearing forces and muscle fibres undergo necrosis and are ultimately replaced with adipose and connective tissue.
Symptoms
The main symptom of Duchenne muscular dystrophy is rapidly progressive muscle weakness associated with muscle wasting with the proximal muscles being first affected, especially the pelvis and calf muscles. Muscle weakness also occurs in the arms, neck, and other areas, but not as severely or as early as in the lower half of the body. Symptoms usually appear before age 6 and may appear as early as infancy. The other physical symptoms are:
- Awkward Gait
- Rapidly progressive
- Frequent falls
- Difficulty with motor skills (running, hopping, jumping)
- Progressive difficulty walking
- Ability to walk is usually lost by the age of 12
- Fatigue
- Mild mental retardation (in approx. 30% of Duchenne's patients)
- Skeletal deformities (including scoliosis in some cases)
- Muscle deformities
- Pseudohypertrophy of tongue and calf muscles. The enlarged muscle tissue is eventually replaced by fat and connective tissue.
- Muscle Contractures of heels and legs, rendering them unusable because the muscle fibers shorten and fibrosis occurs in connective tissue
Signs and tests
Muscle wasting begins in the legs and pelvis, then progresses to the muscles of the shoulders and neck, followed by loss of arm muscles and respiratory muscles. Calf muscle enlargement (pseudohypertrophy) is quite obvious. Cardiomyopathy may occur, but the development of congestive heart failure or arrhythmias (irregular heartbeats) is rare.
- A positive Gower's sign, which reflects the more severe impairment of the lower extremities muscles. The child helps himself to get up with upper extremities: first by rising to stand on his arms and knees, and then "walking" his hands up his legs to stand upright.
- The ability to walk is usually lost by the age of 12.
- Creatine kinase (CPK-MM) levels in the bloodstream are extremely high.
- An electromyography (EMG) shows that weakness is caused by destruction of muscle tissue rather than by damage to nerves.
- Genetic testing
- A muscle biopsy ( immunohistochemistry or immunoblotting) or genetic test ( blood test) confirms the diagnosis.
Treatment
There is no known cure for Duchenne muscular dystrophy. Treatment is aimed at control of symptoms to maximize the quality of life. Physical activity is encouraged. Inactivity (such as bed rest) can worsen the muscle disease. Physical and occupational therapy may be helpful to maintain muscle strength and function. Orthopaedic appliances (such as braces and wheelchairs) may improve mobility and the ability for self-care.
Support Groups
Joining a support group where members share common experiences and problems can often help relieve the stress of this illness. See muscular dystrophy - support group. The Muscular Dystrophy Association (www.mda.org) is an excellent source of information on this disease. Also, the Parent Project http://www.parentprojectmd.org is an excellent source of support and information. There are several pertinent Yahoo groups as well.
Prognosis
Duchenne muscular dystrophy eventually affects all voluntary muscles, and the heart and breathing muscles. Survival is rare beyond the early 30s. Death typically occurs from respiratory failure (suffocation) or heart disorders.
Physiotherapy
Physiotherapists are concerned with enabling children to reach their maximum physical potential. Their aim is to:
- minimize the development of contractures and deformity by developing a programme of stretches and exercises where appropriate
- anticipate and minimise other secondary complications of a physical nature
- prescribe equipment such as orthoses, callipers, wheelchairs and standing frames
- advise on moving and handling issues and equipment
- monitor respiratory function and advise on techniques to assist with breathing exercises and methods of clearing secretions
Mechanical Ventilatory Assistance: Volume Ventilators
Modern "volume ventilators," which deliver a preset volume (amount) of air to the person with each breath, are valuable in the treatment of people with muscular dystrophy related respiratory problems. Ventilator treatment usually begins in childhood when the respiratory muscles begin to fail.
When the vital capacity has dropped below 40 percent of normal, a volume ventilator may be used during sleeping hours, a time when the person is most likely to be underventilating ("hypoventilating"). Hypoventilation during sleep is determined by a thorough history of sleep disorder with an oximetry study and a capillary blood gas (See Pulmonary Function Testing). The ventilator requires a nasal or facemask for connection to the airway. The masks are constructed of comfortable plastic with Velcro straps to hold them in place during sleep.
As the vital capacity declines to less than 30 percent of normal, a volume ventilator may also be needed during the day for more assistance. The person gradually will increase the amount of time using the ventilator during the day as needed. A mouthpiece can be used in the daytime and a nasal or facemask can be used during sleep. The machine can easily fit on a ventilator tray on the bottom of a power wheelchair.
There may be times such as during a respiratory infection when a person needs to rest his/her respiratory muscles during the day even when not yet using full-time ventilation. The versatility of the volume ventilator can meet this need, allowing tired breathing muscles to rest and also allowing aerosol medications to be delivered.
Researching a Cure
Promising research is being conducted around the globe to find a cure, or at the least a therapy that is able to mitigate some of the devastating effects of the disease.
The research group of Kay Davies work on the upregulation of utrophin as a substitute for dystrophin.
At the Généthon Institute in Evry near Paris under Olivier Danos and Luis García the U7 gene transfer technique is under development. This new technique is a combination of exon skipping and the transfer of a gene that instructs the muscle cells to continuously produce the antisense oligonucleotides (AONs) themselves so that they do not have to be injected repeatedly. The AONs are potential drugs which are able to modify the genetic information in such a way that the fast progressing Duchenne muscular dystrophy is converted into the much slower developing Becker muscular dystrophy. Early research into the effects of U7 Gene Transfer have been very promising. Treated mice have gone on to show very little muscle weakness even after being stressed. Treated monkeys have retained the active AONs 6 years after injection, and treated dogs have developed 80% of the normal muscle mass within 2 months of treatment. First round tests in humans are due to begin soon, but given the need for multiple rounds of testing before a treatment can be released to the public, it will be at least a few years before this cure is widely available (if indeed these results are possible in humans).
The U7 gene transfer technique involves delivery of DNA by viral vector into the patient's cells. Other antisense techniques can also modify splicing of pre-mRNA, similarly converting Duchenne to Becker-like muscular dystrophy but without the need for insertion of DNA by virus into the patient. Especially promising for this application are Morpholino antisense oligos , .
More information on the new PTC124 trials is available at the MDA.org website. http://www.mda.org/research/061021dmd_trial_prem_results.html
Prevention
Genetic counseling is advised if there is a family history of the disorder. Duchenne muscular dystrophy can be detected with about 95% accuracy by genetic studies performed during pregnancy.