Arp2/3 complex
2007 Schools Wikipedia Selection. Related subjects: General Biology
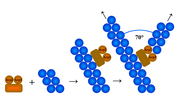
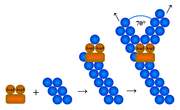
Arp2/3 complex is a seven-subunit protein that plays a major role in the regulation of the actin cytoskeleton. Two of its subunits, the Actin-Related Proteins ARP2 and ARP3 closely resemble the structure of monomeric actin and serve as nucleation sites for new actin filaments. The complex binds to the sides of existing ("mother") filaments and initiates growth of a new ("daughter") filament at a distinctive 70 degree angle from the mother. Branched actin networks are created as a result of this nucleation of new filaments. The regulation of rearrangements of the actin cytoskeleton is important for processes like cell locomotion, phagocytosis, and intracellular motility of lipid vesicles.
The Arp2/3 complex was first identified in Acanthamoeba castellanii and has since been found in every eukaryotic organism studied.
Mechanisms of Actin Polymerization by Arp2/3
Many actin-related molecules create a free barbed end for polymerization by uncapping or severing pre-existing filaments and using these as nucleation cores. However, the Arp2/3 complex stimulates actin polymerization by creating a new nucleation core. The nucleation core activity of Arp2/3 is activated by members of the Wiskott-Aldrich syndrome family protein (WASP, N-WASP, and WAVE proteins). The V domain of a WASP protein interacts with actin monomers while the CA region associates with the Arp2/3 complex to create a nucleation core. However, de novo nucleation followed by polymerization is not sufficient to form integrated actin networks, since these newly synthesized polymers would not be associated with pre-existing filaments. Thus, the Arp2/3 complex binds to pre-existing filaments so that the new filaments can grow on the old ones and form a functional actin cytoskeleton. Capping proteins limit actin polymerization to the region activated by the Arp2/3 complex, and the elongated filament ends are recapped to prevent depolymerization and thus conserve the actin filament.
The Arp2/3 complex simultaneously controls nucleation of actin polymerization and branching of filaments. Moreover, autocatalysis is observed during Arp2/3-mediated actin polymerization. In this process, the newly formed filaments activate other Arp2/3 complexes, facilitating the formation of branched filaments.
The mechanisms of actin polymerization by Arp2/3 has been the subject of dispute in the resent years. The question is where the complex binds the filament and how it nucleates a "daughter" filament. Historically two models have been proposed to describe the formation of branched filaments:
Side branching model
In the side branching (or dendritic nucleation) model, the Arp2/3 complex binds to the side of pre-existing ("mother") filaments at a point different from the nucleation site. Arp2/3 thus has two actin-binding sites — one to bind to the pre-existing actin filament and the other for the nucleation of a branched filament. Recent research provides strong support for this model.
Barbed end branching model
In the barbed end branching model, Arp2/3 associates at the barbed end of growing filaments, allowing for the elongation of the original filament and the formation of a branched filament. This model is mainly based on kinetic analysis rather than structural data, suggesting that branching is induced with Arp2 and Arp3 being incorporated in two different actin filaments.
Cellular Uses of Arp2/3
The Arp2/3 complex appears to be important in a variety of specialized cell functions that involve the actin cytoskeleton. The complex is found in cellular regions characterized by dynamic actin filament activity; in macropinocytotic cups, in the leading edges of lamellipodia, and in motile actin patches in yeast. In mammals and the social amoeba Dictyostelium discoideum it is required for phagocytosis. The complex has also been shown to be involved in the establishment of cell polarity and the migration of fibroblast monolayers in a wound-healing model. Moreover, enteropathogenic organisms like Listeria monocytogenes and Shigella use the Arp2/3 complex for actin-polymerization dependent rocketing movements. The Arp2/3 complex also regulates the intracellular motility of endosomes, lysosomes, pinocytic vesicles and mitochondria. Moreover, recent studies show that the Arp2/3 complex is essential for proper polar cell expansion in plants. Arp2/3 mutations in Arabidopsis result in abnormal filament organization, which in turn affects the expansion of trichomes, pavement cells, hypocotyl cells, and root hair cells.
![Atomic structure of bovine Arp2/3 complex [1] (PDB code: 1k8k). Color coding for subunits: Arp3, orange; Arp2, marine (subunits 1 & 2 not resolved and thus not shown); p40, green; p34, ice blue; p20, dark blue; p21, magenta; p16, yellow.](../../images/166/16654.png)